Early Surgery versus Initial Conservative Treatment in Patients with Traumatic Intracerebral Hemorrhage (STITCH[Trauma]): The First Randomized Trial
- PMID: 25738794
- PMCID: PMC4545564
- DOI: 10.1089/neu.2014.3644
Early Surgery versus Initial Conservative Treatment in Patients with Traumatic Intracerebral Hemorrhage (STITCH[Trauma]): The First Randomized Trial
Abstract
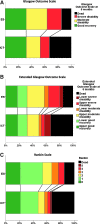
Intraparenchymal hemorrhages occur in a proportion of severe traumatic brain injury TBI patients, but the role of surgery in their treatment is unclear. This international multi-center, patient-randomized, parallel-group trial compared early surgery (hematoma evacuation within 12 h of randomization) with initial conservative treatment (subsequent evacuation allowed if deemed necessary). Patients were randomized using an independent randomization service within 48 h of TBI. Patients were eligible if they had no more than two intraparenchymal hemorrhages of 10 mL or more and did not have an extradural or subdural hematoma that required surgery. The primary outcome measure was the traditional dichotomous split of the Glasgow Outcome Scale obtained by postal questionnaires sent directly to patients at 6 months. The trial was halted early by the UK funding agency (NIHR HTA) for failure to recruit sufficient patients from the UK (trial registration: ISRCTN19321911). A total of 170 patients were randomized from 31 of 59 registered centers worldwide. Of 82 patients randomized to early surgery with complete follow-up, 30 (37%) had an unfavorable outcome. Of 85 patients randomized to initial conservative treatment with complete follow-up, 40 (47%) had an unfavorable outcome (odds ratio, 0.65; 95% confidence interval, CI 0.35, 1.21; p=0.17), with an absolute benefit of 10.5% (CI, -4.4-25.3%). There were significantly more deaths in the first 6 months in the initial conservative treatment group (33% vs. 15%; p=0.006). The 10.5% absolute benefit with early surgery was consistent with the initial power calculation. However, with the low sample size resulting from the premature termination, we cannot exclude the possibility that this could be a chance finding. A further trial is required urgently to assess whether this encouraging signal can be confirmed.
Keywords: controlled trial; craniotomy; intracerebral hemorrhage; randomized; traumatic brain injury.
Figures


References
-
- National Institute for Health and Care Excellence. (2003). NICE clinical guideline sets out recommendations for NHS care of people who have suffered a head injury. National Institute for Health and Care Excellence: London, UK
-
- Fearnside M., and Simpson D. (1997). Epidemiology, in: Head Injury. Reilly P. (ed). Chapman & Hall: London, pps. 1–23
-
- Tagliaferri F., Compagnone C., Korsic M., Servadei F., and Kraus J. (2006). A systematic review of brain injury epidemiology in Europe. Acta Neurochir. (Wien) 148, 255–268; discussion, 268 - PubMed
-
- Gabbe B.J., Biostat G.D., Lecky F.E., Bouamra O., Woodford M., Jenks T., Coats T.J., and Cameron P.A. (2011). The effect of an organized trauma system on mortality in major trauma involving serious head injury: a comparison of the United kingdom and victoria, australia. Ann. Surg. 253, 138–143 - PubMed
-
- Gallbraith S., and Teasdale G. (1981). Predicting the need for operation in the patient with an occult traumatic intracranial hematoma. J. Neurosurg. 55, 75–81 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
