Co-prescription trends in a large cohort of subjects predict substantial drug-drug interactions
- PMID: 25739022
- PMCID: PMC4349653
- DOI: 10.1371/journal.pone.0118991
Co-prescription trends in a large cohort of subjects predict substantial drug-drug interactions
Abstract
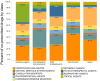
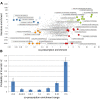
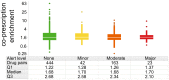
Pharmaceutical prescribing and drug-drug interaction data underlie recommendations on drug combinations that should be avoided or closely monitored by prescribers. Because the number of patients taking multiple medications is increasing, a comprehensive view of prescribing patterns in patients is important to better assess real world pharmaceutical response and evaluate the potential for multi-drug interactions. We obtained self-reported prescription data from NHANES surveys between 1999 and 2010, and confirm the previously reported finding of increasing drug use in the elderly. We studied co-prescription drug trends by focusing on the 2009-2010 survey, which contains prescription data on 690 drugs used by 10,537 subjects. We found that medication profiles were unique for individuals aged 65 years or more, with ≥98 unique drug regimens encountered per 100 subjects taking 3 or more medications. When drugs were viewed by therapeutic class, it was found that the most commonly prescribed drugs were not the most commonly co-prescribed drugs for any of the 16 drug classes investigated. We cross-referenced these medication lists with drug interaction data from Drugs.com to evaluate the potential for drug interactions. The number of drug alerts rose proportionally with the number of co-prescribed medications, rising from 3.3 alerts for individuals prescribed 5 medications to 11.7 alerts for individuals prescribed 10 medications. We found 22% of elderly subjects taking both a substrate and inhibitor of a given cytochrome P450 enzyme, and 4% taking multiple inhibitors of the same enzyme simultaneously. By examining drug pairs prescribed in 0.1% of the population or more, we found low agreement between co-prescription rate and co-discussion in the literature. These data show that prescribing trends in treatment could drive a large extent of individual variability in drug response, and that current pairwise approaches to assessing drug-drug interactions may be inadequate for predicting real world outcomes.
Conflict of interest statement
Figures




References
-
- Center of Disease Control and Prevention. Available: http://www.cdc.gov/nchs/fastats/drugs.htm. Accessed on 30 September 2014.
-
- IMS Health. Available: www.imshealth.com. Accessed on 30 September 2014.
-
- Gu QD, Burt VL. Prescription drug use continues to increase: U.S. prescription drug data for 2007–2008. NCHS Data Brief. 2010; 42: 1–8. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

