Relationship between antibody susceptibility and lipopolysaccharide O-antigen characteristics of invasive and gastrointestinal nontyphoidal Salmonellae isolates from Kenya
- PMID: 25739091
- PMCID: PMC4352093
- DOI: 10.1371/journal.pntd.0003573
Relationship between antibody susceptibility and lipopolysaccharide O-antigen characteristics of invasive and gastrointestinal nontyphoidal Salmonellae isolates from Kenya
Abstract
Background: Nontyphoidal Salmonellae (NTS) cause a large burden of invasive and gastrointestinal disease among young children in sub-Saharan Africa. No vaccine is currently available. Previous reports indicate the importance of the O-antigen of Salmonella lipopolysaccharide for virulence and resistance to antibody-mediated killing. We hypothesised that isolates with more O-antigen have increased resistance to antibody-mediated killing and are more likely to be invasive than gastrointestinal.
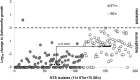
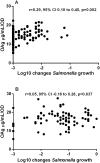
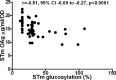
Methodology/principal findings: We studied 192 NTS isolates (114 Typhimurium, 78 Enteritidis) from blood and stools, mostly from paediatric admissions in Kenya 2000-2011. Isolates were tested for susceptibility to antibody-mediated killing, using whole adult serum. O-antigen structural characteristics, including O-acetylation and glucosylation, were investigated. Overall, isolates were susceptible to antibody-mediated killing, but S. Enteritidis were less susceptible and expressed more O-antigen than Typhimurium (p<0.0001 for both comparisons). For S. Typhimurium, but not Enteritidis, O-antigen expression correlated with reduced sensitivity to killing (r = 0.29, 95% CI = 0.10-0.45, p = 0.002). Both serovars expressed O-antigen populations ranging 21-33 kDa average molecular weight. O-antigen from most Typhimurium were O-acetylated on rhamnose and abequose residues, while Enteritidis O-antigen had low or no O-acetylation. Both Typhimurium and Enteritidis O-antigen were approximately 20%-50% glucosylated. Amount of S. Typhimurium O-antigen and O-antigen glucosylation level were inversely related. There was no clear association between clinical presentation and antibody susceptibility, O-antigen level or other O-antigen features.
Conclusion/significance: Kenyan S. Typhimurium and Enteritidis clinical isolates are susceptible to antibody-mediated killing, with degree of susceptibility varying with level of O-antigen for S. Typhimurium. This supports the development of an antibody-inducing vaccine against NTS for Africa. No clear differences were found in the phenotype of isolates from blood and stool, suggesting that the same isolates can cause invasive disease and gastroenteritis. Genome studies are required to understand whether invasive and gastrointestinal isolates differ at the genotypic level.
Conflict of interest statement
FM, LL, RA, AS, CAM, SR are employees of Novartis. AS owns Novartis shares. This does not alter our adherence to all PLOS policies on sharing data and materials.
Figures


References
-
- Vieira A JA, Pires SM, Karlsmose S, Wegener HC, Wong DLF (2009) WHO Global Foodborne Infections Network Country Databank—a resource to link human and non-human sources of Salmonella. Int Soc Vet Epidemiol Econ 643: 512–517.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

