Fabrication of three-dimensionally interconnected nanoparticle superlattices and their lithium-ion storage properties
- PMID: 25739732
- PMCID: PMC4366534
- DOI: 10.1038/ncomms7420
Fabrication of three-dimensionally interconnected nanoparticle superlattices and their lithium-ion storage properties
Abstract
Three-dimensional superlattices consisting of nanoparticles represent a new class of condensed materials with collective properties arising from coupling interactions between close-packed nanoparticles. Despite recent advances in self-assembly of nanoparticle superlattices, the constituent materials have been limited to those that are attainable as monodisperse nanoparticles. In addition, self-assembled nanoparticle superlattices are generally weakly coupled due to the surface-coating ligands. Here we report the fabrication of three-dimensionally interconnected nanoparticle superlattices with face-centered cubic symmetry without the presynthesis of the constituent nanoparticles. We show that mesoporous carbon frameworks derived from self-assembled supercrystals can be used as a robust matrix for the growth of nanoparticle superlattices with diverse compositions. The resulting interconnected nanoparticle superlattices embedded in a carbon matrix are particularly suitable for energy storage applications. We demonstrate this by incorporating tin oxide nanoparticle superlattices as anode materials for lithium-ion batteries, and the resulting electrochemical performance is attributable to their unique architectures.
Figures



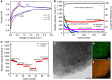
References
-
- Talapin D. V., Lee J.-S., Kovalenko M. V. & Shevchenko E. V. Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev. 110, 389–458 (2010). - PubMed
-
- Panthani M. G. & Korgel B. A. Nanocrystals for electronics. Annu. Rev. Chem. Biomol. Eng. 3, 287–311 (2012). - PubMed
-
- Pileni M. P. Self-assembly of inorganic nanocrystals: fabrication and collective intrinsic properties. Acc. Chem. Res. 40, 685–693 (2007). - PubMed
-
- Murray C. B., Kagan C. R. & Bawendi M. G. Synthesis and characterization of monodisperse nanocrystals and close-packed nanocrystal assemblies. Annu. Rev. Mater. Sci. 30, 545–610 (2000).
-
- Shevchenko E. V., Talapin D. V., Kotov N. A., O'Brien S. & Murray C. B. Structural diversity in binary nanoparticle superlattices. Nature 439, 55–59 (2006). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

