Hypoxia-induced overexpression of stanniocalcin-1 is associated with the metastasis of early stage clear cell renal cell carcinoma
- PMID: 25740019
- PMCID: PMC4337255
- DOI: 10.1186/s12967-015-0421-4
Hypoxia-induced overexpression of stanniocalcin-1 is associated with the metastasis of early stage clear cell renal cell carcinoma
Abstract
Background: Although metastasis of clear cell renal cell carcinoma (ccRCC) is predominantly observed in late stage tumors, early stage metastasis of ccRCC can also be found with indefinite molecular mechanism, leading to inappropriate clinical decisions and poor prognosis. Stanniocalcin-1 (STC1) is a glycoprotein hormone involved in calcium/phosphate homeostasis, which regulates various cellular processes in normal development and tumorigenesis. This study aimed to investigate the role and mechanism of regulation of STC1 in the metastasis of early stage ccRCC.
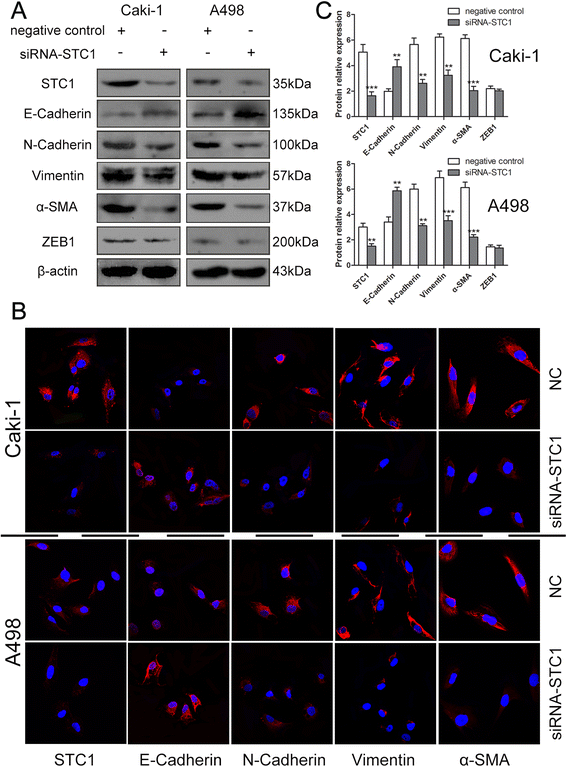
Methods: STC1 mRNA and protein expression was determined in ccRCC surgical specimens, RCC cell lines, and human kidney tubule epithelial cell line HKC by real-time polymerase chain reaction (RT-PCR) and western blotting. Immunohistochemistry staining (IHC) and immunofluorescence were also used to examine the expression and localization of STC1 in ccRCC tissues and cancer cells. Knockdown and overexpression studies were conducted in vitro in RCC cell lines using small interfering RNAs (siRNA) and lentiviral-mediated gene delivery to evaluate the role of STC1 in cell proliferation, anchorage-dependent and independent growth, cell cycle control, and migration and invasion.
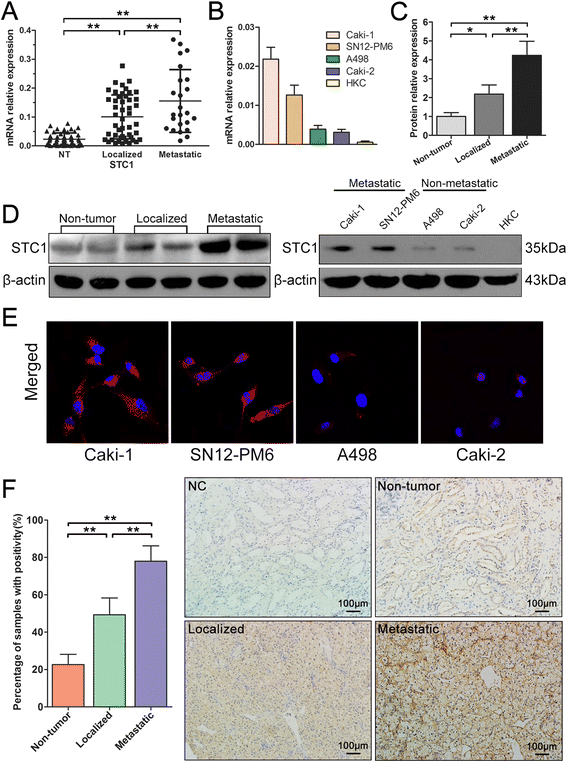
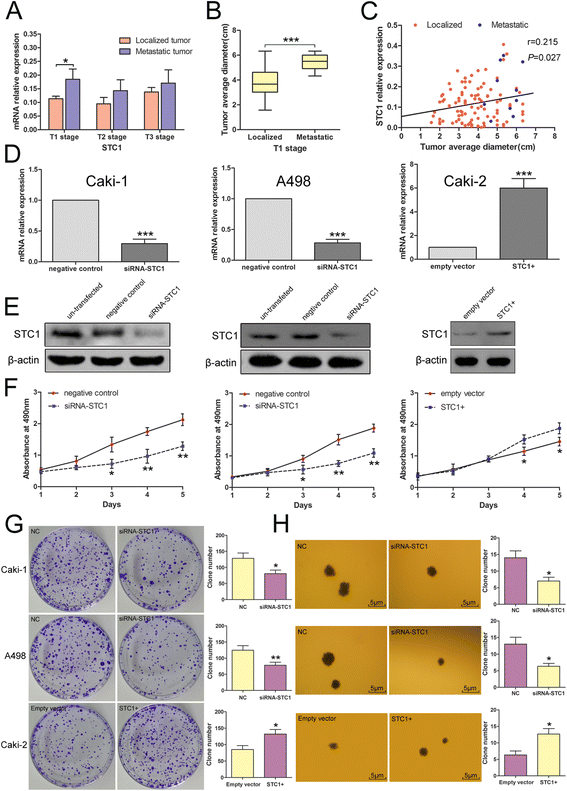
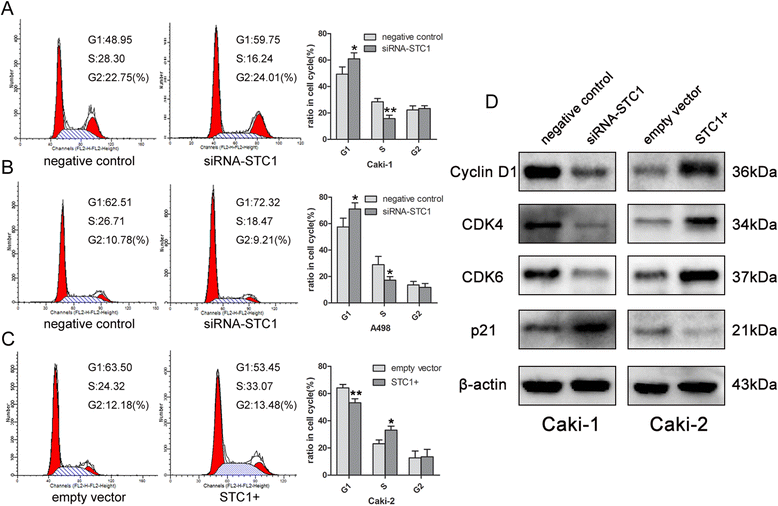
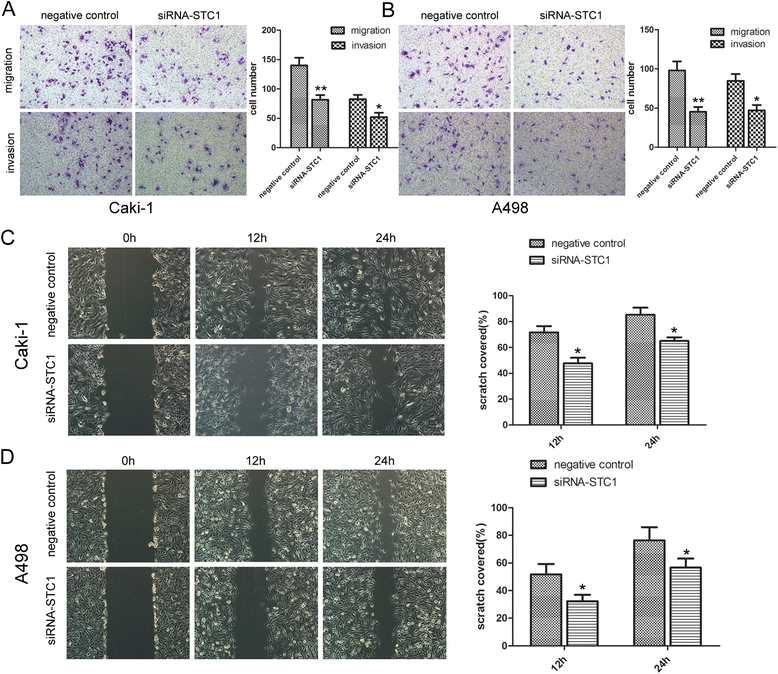
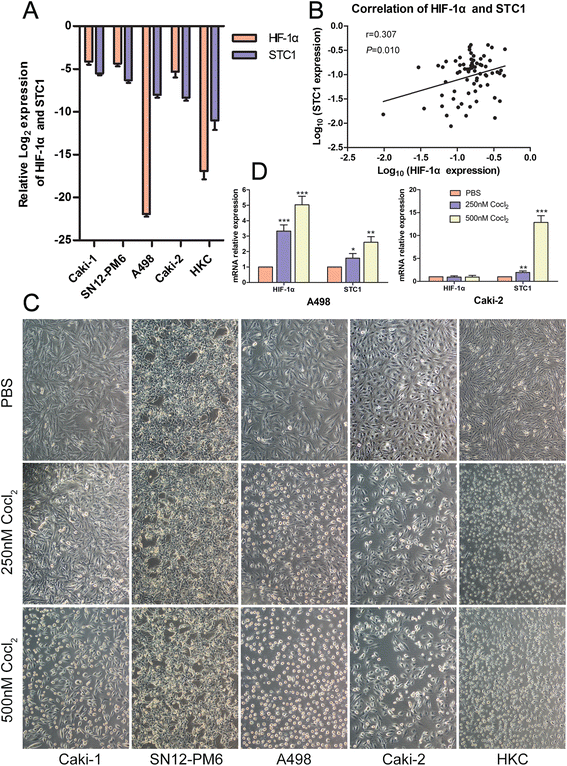
Results: STC1 mRNA and protein expression were significantly up-regulated in tumors when compared with non-tumor tissues, with the greatest increase in expression observed in metastatic tissues. Clinicopathological analysis revealed that STC1 mRNA expression was associated with Fuhrman tumor grade (P = 0.008) and overall Tumor Node Metastasis (TNM) staging (P = 0.018). STC1 expression was elevated in T1 stage metastatic tumors when compared with localized tumors, and was positively correlated with average tumor diameter. Silencing of STC1 expression by Caki-1 and A498 resulted in the inhibition of cell proliferation, migration, and invasion, meanwhile down-regulation of STC1 impaired epithelial-mesenchymal transition (EMT) of ccRCC cell lines. Overexpression of STC1 in Caki-2 enhanced cell growth and proliferation but not migration and invasion. Further investigation identified hypoxia and HIF-1α as candidate regulators of STC1 expression.
Conclusions: Our findings demonstrate a role for STC1 in metastasis of early stage ccRCC and suggest that STC1 may be a biomarker of potential value both for the prognosis of this disease and for guiding clinical decisions regarding surgical strategies and adjuvant treatment.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

