Collagen complexes increase the efficiency of iPS cells generated using fibroblasts from adult mice
- PMID: 25740096
- PMCID: PMC4410313
- DOI: 10.1262/jrd.2014-081
Collagen complexes increase the efficiency of iPS cells generated using fibroblasts from adult mice
Abstract
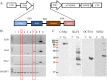
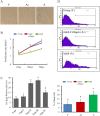
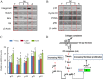
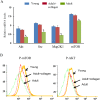
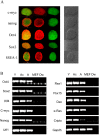
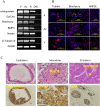
Different interventions are being tested for restoration of the youthfulness of adult mouse-derived fibroblasts. However, fundamental issues, such as the decline of adult mouse-derived fibroblast activity with age, remain unresolved. Therefore, in this study, we examined whether treatment with collagen complexes has beneficial effects on the rejuvenation or reprogramming of adult mouse-derived fibroblasts. Further, we investigated the mechanisms of rejuvenation of adult mouse-derived fibroblasts during treatment with total collagen complexes. We isolated total collagen complexes from the tails of young mice and cultured adult mouse-derived fibroblasts with or without the collagen complexes. When compared with fibroblasts cultured without collagen complexes, adult-derived fibroblasts cultured with collagen complexes over five consecutive passages showed a more youthful state, expanded at a higher rate, and exhibited reduced spontaneous cell death. The fibroblasts cultured in the presence of collagen complexes also showed extensive demethylation in the promoter regions of cell cycle-related genes such as PCNA, increased proliferation, and decreased senescence. In addition, the efficiency of reprogramming of fibroblasts to become induced pluripotent stem (iPS) cells was significantly higher in young- and adult-derived fibroblasts cultured with collagen complexes than in adult-derived fibroblasts cultured alone. Furthermore, mechanistic evidence shows that genes involved in anti-proliferative pathways, including Ink4a/Arf locus genes and p53, were downregulated in fibroblasts exposed to collagen complexes. Interestingly, our results suggest that the rejuvenation process was mediated via the α2β1 integrin-dependent Bmi-1 pathway. Thus, collagen complexes both stimulate proliferation and inhibit cell death and growth arrest in fibroblasts, which appears to be a promising approach for improving the efficiency of reprogramming.
Figures
Similar articles
-
Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells.Stem Cells. 2009 Nov;27(11):2667-74. doi: 10.1002/stem.201. Stem Cells. 2009. PMID: 19697349
-
Generation of mouse induced pluripotent stem cells from different genetic backgrounds using Sleeping beauty transposon mediated gene transfer.Exp Cell Res. 2012 Nov 15;318(19):2482-9. doi: 10.1016/j.yexcr.2012.07.014. Epub 2012 Jul 28. Exp Cell Res. 2012. PMID: 22846649
-
Generation of induced pluripotent stem cells from mouse adipose tissue.Methods Mol Biol. 2014;1194:253-70. doi: 10.1007/978-1-4939-1215-5_14. Methods Mol Biol. 2014. PMID: 25064108
-
[Expansion of MAIT cells via reprogramming to pluripotency and redifferentiation].Seikagaku. 2015 Feb;87(1):112-5. Seikagaku. 2015. PMID: 26571563 Review. Japanese. No abstract available.
-
iPSCs as a major opportunity to understand and cure age-related diseases.Biogerontology. 2015 Aug;16(4):399-410. doi: 10.1007/s10522-015-9579-7. Epub 2015 May 17. Biogerontology. 2015. PMID: 25981448 Review.
Cited by
-
The adaptation of bovine embryonic stem cells to the changes of feeder layers.In Vitro Cell Dev Biol Anim. 2023 Feb;59(2):85-99. doi: 10.1007/s11626-022-00731-5. Epub 2023 Feb 27. In Vitro Cell Dev Biol Anim. 2023. PMID: 36847888
-
Feeder cells treated with ethanol can be used to maintain self-renewal and pluripotency of human pluripotent stem cells.FEBS Open Bio. 2023 Feb;13(2):279-292. doi: 10.1002/2211-5463.13538. Epub 2023 Jan 4. FEBS Open Bio. 2023. PMID: 36537760 Free PMC article.
-
What Kind of Signaling Maintains Pluripotency and Viability in Human-Induced Pluripotent Stem Cells Cultured on Laminin-511 with Serum-Free Medium?Biores Open Access. 2016 Apr 1;5(1):84-93. doi: 10.1089/biores.2016.0001. eCollection 2016. Biores Open Access. 2016. PMID: 27096107 Free PMC article. Review.
References
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126: 663–676. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131: 861–872. - PubMed
-
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007; 318: 1917–1920. - PubMed
-
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol 2008; 26: 101–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

