Early-onset epileptic encephalopathy caused by gain-of-function mutations in the voltage sensor of Kv7.2 and Kv7.3 potassium channel subunits
- PMID: 25740509
- PMCID: PMC6605567
- DOI: 10.1523/JNEUROSCI.4423-14.2015
Early-onset epileptic encephalopathy caused by gain-of-function mutations in the voltage sensor of Kv7.2 and Kv7.3 potassium channel subunits
Abstract
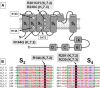
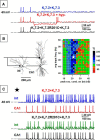
Mutations in Kv7.2 (KCNQ2) and Kv7.3 (KCNQ3) genes, encoding for voltage-gated K(+) channel subunits underlying the neuronal M-current, have been associated with a wide spectrum of early-onset epileptic disorders ranging from benign familial neonatal seizures to severe epileptic encephalopathies. The aim of the present work has been to investigate the molecular mechanisms of channel dysfunction caused by voltage-sensing domain mutations in Kv7.2 (R144Q, R201C, and R201H) or Kv7.3 (R230C) recently found in patients with epileptic encephalopathies and/or intellectual disability. Electrophysiological studies in mammalian cells transfected with human Kv7.2 and/or Kv7.3 cDNAs revealed that each of these four mutations stabilized the activated state of the channel, thereby producing gain-of-function effects, which are opposite to the loss-of-function effects produced by previously found mutations. Multistate structural modeling revealed that the R201 residue in Kv7.2, corresponding to R230 in Kv7.3, stabilized the resting and nearby voltage-sensing domain states by forming an intricate network of electrostatic interactions with neighboring negatively charged residues, a result also confirmed by disulfide trapping experiments. Using a realistic model of a feedforward inhibitory microcircuit in the hippocampal CA1 region, an increased excitability of pyramidal neurons was found upon incorporation of the experimentally defined parameters for mutant M-current, suggesting that changes in network interactions rather than in intrinsic cell properties may be responsible for the neuronal hyperexcitability by these gain-of-function mutations. Together, the present results suggest that gain-of-function mutations in Kv7.2/3 currents may cause human epilepsy with a severe clinical course, thus revealing a previously unexplored level of complexity in disease pathogenetic mechanisms.
Keywords: Kv7 potassium channels; epileptic encephalopathies; gating; mutations; voltage-sensing domain.
Copyright © 2015 the authors 0270-6474/15/353782-12$15.00/0.
Figures





References
-
- Allen AS, Berkovic SF, Cossette P, Delanty N, Dlugos D, Eichler EE, Epstein MP, Glauser T, Goldstein DB, Han Y, Heinzen EL, Hitomi Y, Howell KB, Johnson MR, Kuzniecky R, Lowenstein DH, Lu YF, Madou MR, Marson AG, Mefford HC, et al. De novo mutations in epileptic encephalopathies. Nature. 2013;501:217–221. doi: 10.1038/nature12439. - DOI - PMC - PubMed
-
- Barcia G, Fleming MR, Deligniere A, Gazula VR, Brown MR, Langouet M, Chen H, Kronengold J, Abhyankar A, Cilio R, Nitschke P, Kaminska A, Boddaert N, Casanova JL, Desguerre I, Munnich A, Dulac O, Kaczmarek LK, Colleaux L, Nabbout R. De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nat Genet. 2012;44:1255–1259. doi: 10.1038/ng.2441. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
