Extracellular pH regulates excitability of vomeronasal sensory neurons
- PMID: 25740530
- PMCID: PMC6605571
- DOI: 10.1523/JNEUROSCI.2593-14.2015
Extracellular pH regulates excitability of vomeronasal sensory neurons
Abstract
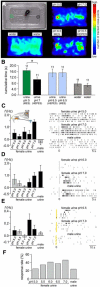
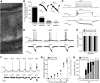
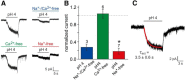
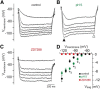
The mouse vomeronasal organ (VNO) plays a critical role in semiochemical detection and social communication. Vomeronasal stimuli are typically secreted in various body fluids. Following direct contact with urine deposits or other secretions, a peristaltic vascular pump mediates fluid entry into the recipient's VNO. Therefore, while vomeronasal sensory neurons (VSNs) sample various stimulatory semiochemicals dissolved in the intraluminal mucus, they might also be affected by the general physicochemical properties of the "solvent." Here, we report cycle stage-correlated variations in urinary pH among female mice. Estrus-specific pH decline is observed exclusively in urine samples from sexually experienced females. Moreover, patch-clamp recordings in acute VNO slices reveal that mouse VSNs reliably detect extracellular acidosis. Acid-evoked responses share the biophysical and pharmacological hallmarks of the hyperpolarization-activated current Ih. Mechanistically, VSN acid sensitivity depends on a pH-induced shift in the voltage-dependence of Ih activation that causes the opening of HCN channels at rest, thereby increasing VSN excitability. Together, our results identify extracellular acidification as a potent activator of vomeronasal Ih and suggest HCN channel-dependent vomeronasal gain control of social chemosignaling. Our data thus reveal a potential mechanistic basis for stimulus pH detection in rodent chemosensory communication.
Keywords: HCN; ion channel; signaling; vomeronasal organ.
Copyright © 2015 the authors 0270-6474/15/354025-15$15.00/0.
Figures






References
-
- Aron C. Mechanisms of control of the reproductive function by olfactory stimuli in female mammals. Physiol Rev. 1979;59:229–284. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
