An out-of-body experience: the extracellular dimension for the transmission of mutualistic bacteria in insects
- PMID: 25740892
- PMCID: PMC4375872
- DOI: 10.1098/rspb.2014.2957
An out-of-body experience: the extracellular dimension for the transmission of mutualistic bacteria in insects
Abstract
Across animals and plants, numerous metabolic and defensive adaptations are a direct consequence of symbiotic associations with beneficial microbes. Explaining how these partnerships are maintained through evolutionary time remains one of the central challenges within the field of symbiosis research. While genome erosion and co-cladogenesis with the host are well-established features of symbionts exhibiting intracellular localization and transmission, the ecological and evolutionary consequences of an extracellular lifestyle have received little attention, despite a demonstrated prevalence and functional importance across many host taxa. Using insect-bacteria symbioses as a model, we highlight the diverse routes of extracellular symbiont transfer. Extracellular transmission routes are unified by the common ability of the bacterial partners to survive outside their hosts, thereby imposing different genomic, metabolic and morphological constraints than would be expected from a strictly intracellular lifestyle. We emphasize that the evolutionary implications of symbiont transmission routes (intracellular versus extracellular) do not necessarily correspond to those of the transmission mode (vertical versus horizontal), a distinction of vital significance when addressing the genomic and physiological consequences for both host and symbiont.
Keywords: host–microbe coevolution; mutualism stability; symbiont transmission; symbiosis.
© 2015 The Author(s) Published by the Royal Society. All rights reserved.
Figures
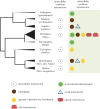

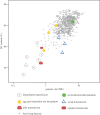
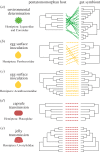
References
-
- Buchner P. 1965. Endosymbiosis of animals with plant microorganisms. New York, NY: Interscience.
-
- Ebert D. 2013. The epidemiology and evolution of symbionts with mixed-mode transmission. Annu. Rev. Ecol. Evol. Syst. 44, 623–643. ( 10.1146/annurev-ecolsys-032513-100555) - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
