Ammonia excretion in Caenorhabditis elegans: mechanism and evidence of ammonia transport of the Rhesus protein CeRhr-1
- PMID: 25740900
- PMCID: PMC4495467
- DOI: 10.1242/jeb.111856
Ammonia excretion in Caenorhabditis elegans: mechanism and evidence of ammonia transport of the Rhesus protein CeRhr-1
Abstract
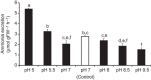
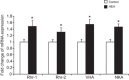
The soil-dwelling nematode Caenorhabditis elegans is a bacteriovorous animal, excreting the vast majority of its nitrogenous waste as ammonia (25.3±1.2 µmol gFW(-1) day(-1)) and very little urea (0.21±0.004 µmol gFW(-1) day(-1)). Although these roundworms have been used for decades as genetic model systems, very little is known about their strategy to eliminate the toxic waste product ammonia from their bodies into the environment. The current study provides evidence that ammonia is at least partially excreted via the hypodermis. Starvation reduced the ammonia excretion rates by more than half, whereas mRNA expression levels of the Rhesus protein CeRhr-2, V-type H(+)-ATPase (subunit A) and Na(+)/K(+)-ATPase (α-subunit) decreased correspondingly. Moreover, ammonia excretion rates were enhanced in media buffered to pH 5 and decreased at pH 9.5. Inhibitor experiments, combined with enzyme activity measurements and mRNA expression analyses, further suggested that the excretion mechanism involves the participation of the V-type H(+)-ATPase, carbonic anhydrase, Na(+)/K(+)-ATPase, and a functional microtubule network. These findings indicate that ammonia is excreted, not only by apical ammonia trapping, but also via vesicular transport and exocytosis. Exposure to 1 mmol l(-1) NH4Cl caused a 10-fold increase in body ammonia and a tripling of ammonia excretion rates. Gene expression levels of CeRhr-1 and CeRhr-2, V-ATPase and Na(+)/K(+)-ATPase also increased significantly in response to 1 mmol l(-1) NH4Cl. Importantly, a functional expression analysis showed, for the first time, ammonia transport capabilities for CeRhr-1 in a phylogenetically ancient invertebrate system, identifying these proteins as potential functional precursors to the vertebrate ammonia-transporting Rh-glycoproteins.
Keywords: Carbonic anhydrase; Na+/K+-ATPase; V-ATPase; Vesicular transport.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures







Similar articles
-
Mechanism of ammonia excretion in the freshwater leech Nephelopsis obscura: characterization of a primitive Rh protein and effects of high environmental ammonia.Am J Physiol Regul Integr Comp Physiol. 2015 Sep 15;309(6):R692-705. doi: 10.1152/ajpregu.00482.2014. Epub 2015 Jul 15. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 26180186 Free PMC article.
-
Ammonia excretion in the freshwater planarian Schmidtea mediterranea.J Exp Biol. 2012 Sep 15;215(Pt 18):3242-53. doi: 10.1242/jeb.067942. Epub 2012 Jun 1. J Exp Biol. 2012. PMID: 22660782
-
Ammonia excretion in the marine polychaete Eurythoe complanata (Annelida).J Exp Biol. 2017 Feb 1;220(Pt 3):425-436. doi: 10.1242/jeb.145615. Epub 2016 Nov 16. J Exp Biol. 2017. PMID: 27852754
-
A new paradigm for ammonia excretion in aquatic animals: role of Rhesus (Rh) glycoproteins.J Exp Biol. 2009 Aug;212(Pt 15):2303-12. doi: 10.1242/jeb.023085. J Exp Biol. 2009. PMID: 19617422 Review.
-
Ammonia excretion in aquatic and terrestrial crabs.J Exp Biol. 2004 Dec;207(Pt 26):4491-504. doi: 10.1242/jeb.01308. J Exp Biol. 2004. PMID: 15579545 Review.
Cited by
-
Development of Aedes aegypti (Diptera: Culicidae) mosquito larvae in high ammonia sewage in septic tanks causes alterations in ammonia excretion, ammonia transporter expression, and osmoregulation.Sci Rep. 2019 Dec 13;9(1):19028. doi: 10.1038/s41598-019-54413-6. Sci Rep. 2019. PMID: 31836747 Free PMC article.
-
Synonymous and Nonsynonymous Substitutions in Dictyostelium discoideum Ammonium Transporter amtA Are Necessary for Functional Complementation in Saccharomyces cerevisiae.Microbiol Spectr. 2023 Feb 22;11(2):e0384722. doi: 10.1128/spectrum.03847-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36840598 Free PMC article.
-
Active mode of excretion across digestive tissues predates the origin of excretory organs.PLoS Biol. 2019 Jul 29;17(7):e3000408. doi: 10.1371/journal.pbio.3000408. eCollection 2019 Jul. PLoS Biol. 2019. PMID: 31356592 Free PMC article.
-
The Reproduction Rate of Peptide Transporter PEPT-1 Deficient C. elegans Is Dependent on Dietary Glutamate Supply.Front Mol Biosci. 2018 Nov 30;5:109. doi: 10.3389/fmolb.2018.00109. eCollection 2018. Front Mol Biosci. 2018. PMID: 30560135 Free PMC article.
-
Mechanism of ammonia excretion in the freshwater leech Nephelopsis obscura: characterization of a primitive Rh protein and effects of high environmental ammonia.Am J Physiol Regul Integr Comp Physiol. 2015 Sep 15;309(6):R692-705. doi: 10.1152/ajpregu.00482.2014. Epub 2015 Jul 15. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 26180186 Free PMC article.
References
-
- Brady N. C. and Weil R. R. (2008). The Nature and Properties of Soils. Columbus, OH, USA: Prentice Hall Publishing.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

