Pregnancy Does Not Attenuate the Antibody or Plasmablast Response to Inactivated Influenza Vaccine
- PMID: 25740957
- PMCID: PMC4548461
- DOI: 10.1093/infdis/jiv138
Pregnancy Does Not Attenuate the Antibody or Plasmablast Response to Inactivated Influenza Vaccine
Abstract
Background: Inactivated influenza vaccine (IIV) is recommended during pregnancy to prevent influenza infection and its complications in pregnant women and their infants. However, the extent to which pregnancy modifies the antibody response to vaccination remains unclear, and prior studies have focused primarily on hemagglutinin inhibition (HI) titers. A more comprehensive understanding of how pregnancy modifies the humoral immune response to influenza vaccination will aid in maximizing vaccine efficacy.
Methods: Healthy pregnant women and control women were studied prior to, 7 days after, and 28 days after vaccination with IIV. HI titers, microneutralization (MN) titers, and the frequency of circulating plasmablasts were evaluated in pregnant versus control women.
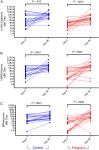
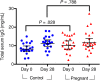
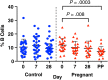
Results: Pregnant women and control women mount similarly robust serologic immune responses to IIV, with no significant differences for any influenza strain in postvaccination geometric mean HI or MN titers. HI and MN titers correlate, though MN titers demonstrate more robust changes pre- versus postvaccination. The induction of circulating plasmablasts is increased in pregnant women versus controls (median fold-change 2.60 vs 1.49 [interquartile range, 0.94-7.53 vs 0.63-2.67]; P = .03).
Conclusions: Pregnant women do not have impaired humoral immune responses to IIV and may have increased circulating plasmablast production compared to control women.
Keywords: hemagglutinin inhibition; influenza; plasmablast; pregnancy; vaccination; viral neutralization.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Gabriel G, Arck PC. Sex, immunity and influenza. J Infect Dis 2014; 209(Suppl 3):S93–9. - PubMed
-
- Harris JW. Influenza occurring in pregnant women: a statistical study of thirteen hundred and fifty cases. JAMA 1919; 72:978–80.
-
- Jamieson DJ, Honein MA, Rasmussen SA, et al. Articles. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009; 374:451–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

