From two competing oscillators to one coupled-clock pacemaker cell system
- PMID: 25741284
- PMCID: PMC4327306
- DOI: 10.3389/fphys.2015.00028
From two competing oscillators to one coupled-clock pacemaker cell system
Abstract
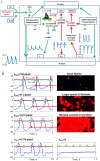
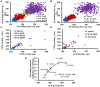
At the beginning of this century, debates regarding "what are the main control mechanisms that ignite the action potential (AP) in heart pacemaker cells" dominated the electrophysiology field. The original theory which prevailed for over 50 years had advocated that the ensemble of surface membrane ion channels (i.e., "M-clock") is sufficient to ignite rhythmic APs. However, more recent experimental evidence in a variety of mammals has shown that the sarcoplasmic reticulum (SR) acts as a "Ca(2+)-clock" rhythmically discharges diastolic local Ca(2+) releases (LCRs) beneath the cell surface membrane. LCRs activate an inward current (likely that of the Na(+)/Ca(2+) exchanger) that prompts the surface membrane "M-clock" to ignite an AP. Theoretical and experimental evidence has mounted to indicate that this clock "crosstalk" operates on a beat-to-beat basis and determines both the AP firing rate and rhythm. Our review is focused on the evolution of experimental definition and numerical modeling of the coupled-clock concept, on how mechanisms intrinsic to pacemaker cell determine both the heart rate and rhythm, and on future directions to develop further the coupled-clock pacemaker cell concept.
Keywords: arrhythmias; coupled-clock pacemaker system; heart rate variability; mathematical modeling; sinoatrial node.
Figures
Similar articles
-
Stochasticity intrinsic to coupled-clock mechanisms underlies beat-to-beat variability of spontaneous action potential firing in sinoatrial node pacemaker cells.J Mol Cell Cardiol. 2014 Dec;77:1-10. doi: 10.1016/j.yjmcc.2014.09.008. Epub 2014 Sep 22. J Mol Cell Cardiol. 2014. PMID: 25257916 Free PMC article.
-
New evidence for coupled clock regulation of the normal automaticity of sinoatrial nodal pacemaker cells: bradycardic effects of ivabradine are linked to suppression of intracellular Ca²⁺ cycling.J Mol Cell Cardiol. 2013 Sep;62:80-9. doi: 10.1016/j.yjmcc.2013.04.026. Epub 2013 May 5. J Mol Cell Cardiol. 2013. PMID: 23651631 Free PMC article.
-
Positive Feedback Mechanisms among Local Ca Releases, NCX, and ICaL Ignite Pacemaker Action Potentials.Biophys J. 2018 Mar 13;114(5):1176-1189. doi: 10.1016/j.bpj.2017.12.043. Biophys J. 2018. PMID: 29539403 Free PMC article.
-
Dynamic interactions of an intracellular Ca2+ clock and membrane ion channel clock underlie robust initiation and regulation of cardiac pacemaker function.Cardiovasc Res. 2008 Jan 15;77(2):274-84. doi: 10.1093/cvr/cvm058. Epub 2007 Nov 5. Cardiovasc Res. 2008. PMID: 18006441 Review.
-
The emergence of a general theory of the initiation and strength of the heartbeat.J Pharmacol Sci. 2006;100(5):338-69. doi: 10.1254/jphs.cr0060018. J Pharmacol Sci. 2006. PMID: 16799255 Review.
Cited by
-
The end effector of circadian heart rate variation: the sinoatrial node pacemaker cell.BMB Rep. 2015 Dec;48(12):677-84. doi: 10.5483/bmbrep.2015.48.12.061. BMB Rep. 2015. PMID: 25999176 Free PMC article. Review.
-
Principles, mechanisms and functions of entrainment in biological oscillators.Interface Focus. 2022 Apr 15;12(3):20210088. doi: 10.1098/rsfs.2021.0088. eCollection 2022 Jun 6. Interface Focus. 2022. PMID: 35450280 Free PMC article. Review.
-
Conditional ablation and conditional rescue models for Casq2 elucidate the role of development and of cell-type specific expression of Casq2 in the CPVT2 phenotype.Hum Mol Genet. 2018 May 1;27(9):1533-1544. doi: 10.1093/hmg/ddy060. Hum Mol Genet. 2018. PMID: 29452352 Free PMC article.
-
Molecular Basis of Atrial Fibrillation Pathophysiology and Therapy: A Translational Perspective.Circ Res. 2020 Jun 19;127(1):51-72. doi: 10.1161/CIRCRESAHA.120.316363. Epub 2020 Jun 18. Circ Res. 2020. PMID: 32717172 Free PMC article. Review.
-
Modelling the coupling of the M-clock and C-clock in lymphatic muscle cells.Comput Biol Med. 2022 Mar;142:105189. doi: 10.1016/j.compbiomed.2021.105189. Epub 2021 Dec 29. Comput Biol Med. 2022. PMID: 34995957 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

