Cellular hyper-excitability caused by mutations that alter the activation process of voltage-gated sodium channels
- PMID: 25741286
- PMCID: PMC4330716
- DOI: 10.3389/fphys.2015.00045
Cellular hyper-excitability caused by mutations that alter the activation process of voltage-gated sodium channels
Abstract
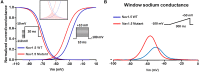
Voltage-gated sodium channels (Nav) are widely expressed as macro-molecular complexes in both excitable and non-excitable tissues. In excitable tissues, the upstroke of the action potential is the result of the passage of a large and rapid influx of sodium ions through these channels. NaV dysfunction has been associated with an increasingly wide range of neurological, muscular and cardiac disorders. The purpose of this review is to summarize the recently identified sodium channel mutations that are linked to hyper-excitability phenotypes and associated with the alteration of the activation process of voltage gated sodium channels. Indeed, several clinical manifestations that demonstrate an alteration of tissue excitability were recently shown to be strongly associated with the presence of mutations that affect the activation process of the Nav. These emerging genotype-phenotype correlations have expanded the clinical spectrum of sodium channelopathies to include disorders which feature a hyper-excitability phenotype that may or may not be associated with a cardiomyopathy. The p.I141V mutation in SCN4A and SCN5A, as well as its homologous p.I136V mutation in SCN9A, are interesting examples of mutations that have been linked to inherited hyperexcitability myotonia, exercise-induced polymorphic ventricular arrhythmias and erythromelalgia, respectively. Regardless of which sodium channel isoform is investigated, the substitution of the isoleucine to valine in the locus 141 induces similar modifications in the biophysical properties of the Nav by shifting the voltage-dependence of steady state activation toward more negative potentials.
Keywords: Nav1.5-I141V; dilated cardiomyopathy; erythromelalgia; hyper-excitability; myotonia.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

