Drug resistance in cortical and hippocampal slices from resected tissue of epilepsy patients: no significant impact of p-glycoprotein and multidrug resistance-associated proteins
- PMID: 25741317
- PMCID: PMC4332373
- DOI: 10.3389/fneur.2015.00030
Drug resistance in cortical and hippocampal slices from resected tissue of epilepsy patients: no significant impact of p-glycoprotein and multidrug resistance-associated proteins
Abstract
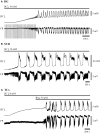
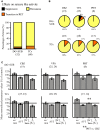
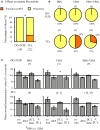
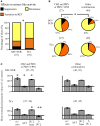
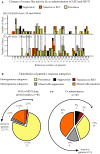
Drug resistant patients undergoing epilepsy surgery have a good chance to become sensitive to anticonvulsant medication, suggesting that the resected brain tissue is responsible for drug resistance. Here, we address the question whether P-glycoprotein (Pgp) and multidrug resistance-associated proteins (MRPs) expressed in the resected tissue contribute to drug resistance in vitro. Effects of anti-epileptic drugs [carbamazepine (CBZ), sodium valproate, phenytoin] and two unspecific inhibitors of Pgp and MRPs [verapamil (VPM) and probenecid (PBN)] on seizure-like events (SLEs) induced in slices from 35 hippocampal and 35 temporal cortex specimens of altogether 51 patients (161 slices) were studied. Although in slice preparations the blood brain barrier is not functional, we found that SLEs predominantly persisted in the presence of anticonvulsant drugs (90%) and also in the presence of VPM and PBN (86%). Following subsequent co-administration of anti-epileptic drugs and drug transport inhibitors, SLEs continued in 63% of 143 slices. Drug sensitivity in slices was recognized either as transition to recurrent epileptiform transients (30%) or as suppression (7%), particularly by perfusion with CBZ in PBN containing solutions (43, 9%). Summarizing responses to co-administration from more than one slice per patient revealed that suppression of seizure-like activity in all slices was only observed in 7% of patients. Patients whose tissue was completely or partially sensitive (65%) presented with higher seizure frequencies than those with resistant tissue (35%). However, corresponding subgroups of patients do not differ with respect to expression rates of drug transporters. Our results imply that parenchymal MRPs and Pgp are not responsible for drug resistance in resected tissue.
Keywords: TLE; carbamazepine; phenytoin; probenecid; seizure-like events; sodium valproate; surgically resected tissue; verapamil.
Figures





Similar articles
-
Carbamazepine-resistance in the epileptic dentate gyrus of human hippocampal slices.Brain. 2006 Dec;129(Pt 12):3290-306. doi: 10.1093/brain/awl218. Epub 2006 Sep 2. Brain. 2006. PMID: 16951410
-
Functional test of multidrug transporter activity in hippocampal-neocortical brain slices from epileptic patients.J Neurosci Methods. 2011 Sep 15;200(2):164-72. doi: 10.1016/j.jneumeth.2011.06.032. Epub 2011 Jul 7. J Neurosci Methods. 2011. PMID: 21763346
-
P-glycoprotein and multidrug resistance-associated protein are involved in the regulation of extracellular levels of the major antiepileptic drug carbamazepine in the brain.Neuroreport. 2001 Nov 16;12(16):3557-60. doi: 10.1097/00001756-200111160-00037. Neuroreport. 2001. PMID: 11733711
-
P-glycoprotein imaging in temporal lobe epilepsy: in vivo PET experiments with the Pgp substrate [11C]-verapamil.Epilepsia. 2012 Nov;53 Suppl 6:60-3. doi: 10.1111/j.1528-1167.2012.03704.x. Epilepsia. 2012. PMID: 23134497 Review.
-
ABC transporters during epilepsy and mechanisms underlying multidrug resistance in refractory epilepsy.Epilepsia. 2007;48 Suppl 5:140-9. doi: 10.1111/j.1528-1167.2007.01302.x. Epilepsia. 2007. PMID: 17910594 Review.
Cited by
-
Different patterns of epileptiform-like activity are generated in the sclerotic hippocampus from patients with drug-resistant temporal lobe epilepsy.Sci Rep. 2018 May 8;8(1):7116. doi: 10.1038/s41598-018-25378-9. Sci Rep. 2018. PMID: 29740014 Free PMC article.
-
DREADDs suppress seizure-like activity in a mouse model of pharmacoresistant epileptic brain tissue.Gene Ther. 2016 Oct;23(10):760-766. doi: 10.1038/gt.2016.56. Epub 2016 Jul 14. Gene Ther. 2016. PMID: 27416078
-
Carbamazepine modulates the spatiotemporal activity in the dentate gyrus of rats and pharmacoresistant humans in vitro.Brain Behav. 2016 Apr 15;6(6):e00463. doi: 10.1002/brb3.463. eCollection 2016 Jun. Brain Behav. 2016. PMID: 27110437 Free PMC article.
-
Immunotherapy by targeting of VGKC complex for seizure control and prevention of cognitive impairment in a mouse model of epilepsy.Mol Med Rep. 2018 Jul;18(1):169-178. doi: 10.3892/mmr.2018.9004. Epub 2018 May 9. Mol Med Rep. 2018. PMID: 29749462 Free PMC article.
-
Human Cerebrospinal Fluid Induces Neuronal Excitability Changes in Resected Human Neocortical and Hippocampal Brain Slices.Front Neurosci. 2020 Apr 21;14:283. doi: 10.3389/fnins.2020.00283. eCollection 2020. Front Neurosci. 2020. PMID: 32372899 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

