Malaria and the liver: immunological hide-and-seek or subversion of immunity from within?
- PMID: 25741320
- PMCID: PMC4332352
- DOI: 10.3389/fmicb.2015.00041
Malaria and the liver: immunological hide-and-seek or subversion of immunity from within?
Erratum in
-
Corrigendum: Malaria and the liver: immunological hide-and-seek or subversion of immunity from within?Front Microbiol. 2015 May 13;6:460. doi: 10.3389/fmicb.2015.00460. eCollection 2015. Front Microbiol. 2015. PMID: 26029194 Free PMC article.
Abstract
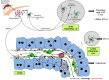
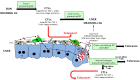
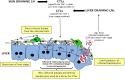
During the pre-erythrocytic asymptomatic phase of malarial infection, sporozoites develop transiently inside less than 100 hepatocytes that subsequently release thousands of merozoites. Killing of these hepatocytes by cytotoxic T cells (CTLs) confers protection to subsequent malarial infection, suggesting that this bottleneck phase in the parasite life cycle can be targeted by vaccination. During natural transmission, although some CTLs are generated in the skin draining lymph nodes, they are unable to eliminate the parasite, suggesting that the liver is important for the sporozoite to escape immune surveillance. The contribution of the organ to this process is unclear. Based on the known ability of several hepatic antigen-presenting cells (APCs) to induce primary activation of CD8 T cells and tolerance, malarial antigens presented by both infected hepatocytes and/or hepatic cross-presenting APCs should result in tolerance. However, our latest model predicts that due to the low frequency of infected hepatocytes, some T cells recognizing sporozoite epitopes with high affinity should differentiate into CTLs. In this review, we discuss two possible models to explain why CTLs generated in the liver and skin draining lymph nodes are unable to eliminate the parasite: (1) sporozoites harness the tolerogenic property of the liver; (2) CTLs are not tolerized but fail to detect infected cells due to sparse infection of hepatocytes and the very short liver stage. We propose that while malaria sporozoites might use the ability of the liver to tolerize both naive and effector cells, they have also developed strategies to decrease the probability of encounter between CTLs and infected liver cells. Thus, we predict that to achieve protection, vaccination strategies should aim to boost intrahepatic activation and/or increase the chance of encounter between sporozoite-specific CTLs and infected hepatocytes.
Keywords: Kupffer cells; LSEC; T cells; dendritic cells; hepatic stellate cells; hepatocytes; sporozoite; tolerance.
Figures
Similar articles
-
Genetically attenuated parasite vaccines induce contact-dependent CD8+ T cell killing of Plasmodium yoelii liver stage-infected hepatocytes.J Immunol. 2009 Nov 1;183(9):5870-8. doi: 10.4049/jimmunol.0900302. Epub 2009 Oct 7. J Immunol. 2009. PMID: 19812194
-
Tissue signatures influence the activation of intrahepatic CD8(+) T cells against malaria sporozoites.Front Microbiol. 2014 Aug 22;5:440. doi: 10.3389/fmicb.2014.00440. eCollection 2014. Front Microbiol. 2014. PMID: 25202304 Free PMC article. Review.
-
The Threshold of Protection from Liver-Stage Malaria Relies on a Fine Balance between the Number of Infected Hepatocytes and Effector CD8+ T Cells Present in the Liver.J Immunol. 2017 Mar 1;198(5):2006-2016. doi: 10.4049/jimmunol.1601209. Epub 2017 Jan 13. J Immunol. 2017. PMID: 28087668 Free PMC article.
-
Defining rules of CD8(+) T cell expansion against pre-erythrocytic Plasmodium antigens in sporozoite-immunized mice.Malar J. 2016 Apr 26;15:238. doi: 10.1186/s12936-016-1295-5. Malar J. 2016. PMID: 27113469 Free PMC article.
-
Priming of CD8(+) T Cell Responses to Liver Stage Malaria Parasite Antigens.Front Immunol. 2014 Nov 5;5:527. doi: 10.3389/fimmu.2014.00527. eCollection 2014. Front Immunol. 2014. PMID: 25414698 Free PMC article. Review.
Cited by
-
Host-parasite interactions during Plasmodium infection: Implications for immunotherapies.Front Immunol. 2023 Jan 4;13:1091961. doi: 10.3389/fimmu.2022.1091961. eCollection 2022. Front Immunol. 2023. PMID: 36685595 Free PMC article. Review.
-
Delayed fractional dose regimen of the RTS,S/AS01 malaria vaccine candidate enhances an IgG4 response that inhibits serum opsonophagocytosis.Sci Rep. 2017 Aug 11;7(1):7998. doi: 10.1038/s41598-017-08526-5. Sci Rep. 2017. PMID: 28801554 Free PMC article. Clinical Trial.
-
Protective Vaccination against Blood-Stage Malaria of Plasmodium chabaudi: Differential Gene Expression in the Liver of Balb/c Mice toward the End of Crisis Phase.Front Microbiol. 2016 Jul 14;7:1087. doi: 10.3389/fmicb.2016.01087. eCollection 2016. Front Microbiol. 2016. PMID: 27471498 Free PMC article.
-
Role of Opsonophagocytosis in Immune Protection against Malaria.Vaccines (Basel). 2020 May 30;8(2):264. doi: 10.3390/vaccines8020264. Vaccines (Basel). 2020. PMID: 32486320 Free PMC article. Review.
-
A prime-boost immunization regimen based on a simian adenovirus 36 vectored multi-stage malaria vaccine induces protective immunity in mice.Vaccine. 2017 May 31;35(24):3239-3248. doi: 10.1016/j.vaccine.2017.04.062. Epub 2017 May 5. Vaccine. 2017. PMID: 28483199 Free PMC article.
References
-
- Beattie L., Peltan A., Maroof A., Kirby A., Brown N., Coles M., et al. . (2010). Dynamic imaging of experimental Leishmania donovani-induced hepatic granulomas detects Kupffer cell-restricted antigen presentation to antigen-specific CD8 T cells. PLoS Pathog. 6:e1000805. 10.1371/journal.ppat.1000805 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

