Characterization of the E. coli proteome and its modifications during growth and ethanol stress
- PMID: 25741329
- PMCID: PMC4332353
- DOI: 10.3389/fmicb.2015.00103
Characterization of the E. coli proteome and its modifications during growth and ethanol stress
Abstract
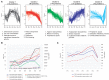
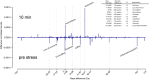
We set out to provide a resource to the microbiology community especially with respect to systems biology based endeavors. To this end, we generated a comprehensive dataset monitoring the changes in protein expression, copy number, and post translational modifications in a systematic fashion during growth and ethanol stress in E. coli. We utilized high-resolution mass spectrometry (MS) combined with the Super-SILAC approach. In a single experiment, we have identified over 2300 proteins, which represent approximately 88% of the estimated expressed proteome of E. coli and estimated protein copy numbers using the Intensity Based Absolute Quantitation (iBAQ). The dynamic range of protein expression spanned up to six orders of magnitude, with the highest protein copy per cell estimated at approximately 300,000. We focused on the proteome dynamics involved during stationary phase growth. A global up-regulation of proteins related to stress response was detected in later stages of growth. We observed the down-regulation of the methyl directed mismatch repair system containing MutS and MutL of E. coli growing in long term growth cultures, confirming that higher incidence of mutations presents an important mechanism in the increase in genetic diversity and stationary phase survival in E. coli. During ethanol stress, known markers such as alcohol dehydrogenase and aldehyde dehydrogenase were induced, further validating the dataset. Finally, we performed unbiased protein modification detection and revealed changes of many known and unknown protein modifications in both experimental conditions. Data are available via ProteomeXchange with identifier PXD001648.
Keywords: E. coli; Super-SILAC; absolute quantitation; post translational modifications; quantitative proteomics; stress response.
Figures


References
-
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Statist. Soc. B 57, 289–300.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

