Enriching distinctive microbial communities from marine sediments via an electrochemical-sulfide-oxidizing process on carbon electrodes
- PMID: 25741331
- PMCID: PMC4330880
- DOI: 10.3389/fmicb.2015.00111
Enriching distinctive microbial communities from marine sediments via an electrochemical-sulfide-oxidizing process on carbon electrodes
Abstract
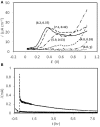
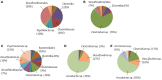
Sulfide is a common product of marine anaerobic respiration, and a potent reactant biologically and geochemically. Here we demonstrate the impact on microbial communities with the removal of sulfide via electrochemical methods. The use of differential pulse voltammetry revealed that the oxidation of soluble sulfide was seen at +30 mV (vs. SHE) at all pH ranges tested (from pH = 4 to 8), while non-ionized sulfide, which dominated at pH = 4 was poorly oxidized via this process. Two mixed cultures (CAT and LA) were enriched from two different marine sediments (from Catalina Island, CAT; from the Port of Los Angeles, LA) in serum bottles using a seawater medium supplemented with lactate, sulfate, and yeast extract, to obtain abundant biomass. Both CAT and LA cultures were inoculated in electrochemical cells (using yeast-extract-free seawater medium as an electrolyte) equipped with carbon-felt electrodes. In both cases, when potentials of +630 or +130 mV (vs. SHE) were applied, currents were consistently higher at +630 then at +130 mV, indicating more sulfide being oxidized at the higher potential. In addition, higher organic-acid and sulfate conversion rates were found at +630 mV with CAT, while no significant differences were found with LA at different potentials. The results of microbial-community analyses revealed a decrease in diversity for both CAT and LA after electrochemical incubation. In addition, some bacteria (e.g., Clostridium and Arcobacter) not well-known to be capable of extracellular electron transfer, were found to be dominant in the electrochemical cells. Thus, even though the different mixed cultures have different tolerances for sulfide, electrochemical-sulfide removal can lead to major population changes.
Keywords: carbon electrodes; differential pulse voltammetry; electrochemical sulfide oxidation; marine sediments; microbial community analyses.
Figures


Similar articles
-
Anode potential selection for sulfide removal in contaminated marine sediments.J Hazard Mater. 2018 Oct 15;360:498-503. doi: 10.1016/j.jhazmat.2018.08.016. Epub 2018 Aug 16. J Hazard Mater. 2018. PMID: 30145477
-
Arcobacter peruensis sp. nov., a Chemolithoheterotroph Isolated from Sulfide- and Organic-Rich Coastal Waters off Peru.Appl Environ Microbiol. 2019 Nov 27;85(24):e01344-19. doi: 10.1128/AEM.01344-19. Print 2019 Dec 15. Appl Environ Microbiol. 2019. PMID: 31585991 Free PMC article.
-
Microbial Diversity in Sulfate-Reducing Marine Sediment Enrichment Cultures Associated with Anaerobic Biotransformation of Coastal Stockpiled Phosphogypsum (Sfax, Tunisia).Front Microbiol. 2017 Aug 21;8:1583. doi: 10.3389/fmicb.2017.01583. eCollection 2017. Front Microbiol. 2017. PMID: 28871244 Free PMC article.
-
Differences in Applied Redox Potential on Cathodes Enrich for Diverse Electrochemically Active Microbial Isolates From a Marine Sediment.Front Microbiol. 2019 Aug 28;10:1979. doi: 10.3389/fmicb.2019.01979. eCollection 2019. Front Microbiol. 2019. PMID: 31555224 Free PMC article.
-
Current approaches for mitigating acid mine drainage.Rev Environ Contam Toxicol. 2013;226:1-32. doi: 10.1007/978-1-4614-6898-1_1. Rev Environ Contam Toxicol. 2013. PMID: 23625128 Review.
Cited by
-
Assessing Marine Microbial Induced Corrosion at Santa Catalina Island, California.Front Microbiol. 2016 Oct 25;7:1679. doi: 10.3389/fmicb.2016.01679. eCollection 2016. Front Microbiol. 2016. PMID: 27826293 Free PMC article.
-
Responses of electroactive biofilms to chronic chlorine exposure: Insights from the composition and spatial structure of extracellular polymeric substances.Bioelectrochemistry. 2021 Dec;142:107894. doi: 10.1016/j.bioelechem.2021.107894. Epub 2021 Jul 27. Bioelectrochemistry. 2021. PMID: 34371350 Free PMC article.
-
Studies on expression levels of pil Q and fli P genes during bio-electrogenic process in Kluyvera georgiana MCC 3673.3 Biotech. 2020 Feb;10(2):73. doi: 10.1007/s13205-020-2050-8. Epub 2020 Jan 28. 3 Biotech. 2020. PMID: 32051806 Free PMC article.
-
Wiring Up Along Electrodes for Biofilm Formation.Front Microbiol. 2021 Aug 30;12:726251. doi: 10.3389/fmicb.2021.726251. eCollection 2021. Front Microbiol. 2021. PMID: 34526980 Free PMC article.
References
-
- Brett C. M. A., Brett A. M. O. (1993). Electrochemistry: Principles, Methods, and Applications. Oxford: Oxford Uniersity Press.
-
- Burdige D. J. (2006). Geochemistry of Marine Sediments. Princeton, NJ: Princeton University Press.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

