Partial restoration of protein synthesis rates by the small molecule ISRIB prevents neurodegeneration without pancreatic toxicity
- PMID: 25741597
- PMCID: PMC4385927
- DOI: 10.1038/cddis.2015.49
Partial restoration of protein synthesis rates by the small molecule ISRIB prevents neurodegeneration without pancreatic toxicity
Abstract
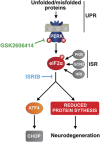
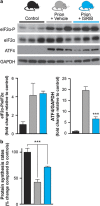
Activation of the PERK branch of the unfolded protein response (UPR) in response to protein misfolding within the endoplasmic reticulum (ER) results in the transient repression of protein synthesis, mediated by the phosphorylation of the alpha subunit of eukaryotic initiation factor 2 (eIF2α). This is part of a wider integrated physiological response to maintain proteostasis in the face of ER stress, the dysregulation of which is increasingly associated with a wide range of diseases, particularly neurodegenerative disorders. In prion-diseased mice, persistently high levels of eIF2α cause sustained translational repression leading to catastrophic reduction of critical proteins, resulting in synaptic failure and neuronal loss. We previously showed that restoration of global protein synthesis using the PERK inhibitor GSK2606414 was profoundly neuroprotective, preventing clinical disease in prion-infected mice. However, this occured at the cost of toxicity to secretory tissue, where UPR activation is essential to healthy functioning. Here we show that pharmacological modulation of eIF2α-P-mediated translational inhibition can be achieved to produce neuroprotection without pancreatic toxicity. We found that treatment with the small molecule ISRIB, which restores translation downstream of eIF2α, conferred neuroprotection in prion-diseased mice without adverse effects on the pancreas. Critically, ISRIB treatment resulted in only partial restoration of global translation rates, as compared with the complete restoration of protein synthesis seen with GSK2606414. ISRIB likely provides sufficient rates of protein synthesis for neuronal survival, while allowing some residual protective UPR function in secretory tissue. Thus, fine-tuning the extent of UPR inhibition and subsequent translational de-repression uncouples neuroprotective effects from pancreatic toxicity. The data support the pursuit of this approach to develop new treatments for a range of neurodegenerative disorders that are currently incurable.
Figures




Similar articles
-
Fine tuning of the unfolded protein response by ISRIB improves neuronal survival in a model of amyotrophic lateral sclerosis.Cell Death Dis. 2020 May 26;11(5):397. doi: 10.1038/s41419-020-2601-2. Cell Death Dis. 2020. PMID: 32457286 Free PMC article.
-
PERK inhibition prevents tau-mediated neurodegeneration in a mouse model of frontotemporal dementia.Acta Neuropathol. 2015 Nov;130(5):633-42. doi: 10.1007/s00401-015-1487-z. Epub 2015 Oct 8. Acta Neuropathol. 2015. PMID: 26450683 Free PMC article.
-
Sustained translational repression by eIF2α-P mediates prion neurodegeneration.Nature. 2012 May 6;485(7399):507-11. doi: 10.1038/nature11058. Nature. 2012. PMID: 22622579 Free PMC article.
-
The UPR and synaptic dysfunction in neurodegeneration.Brain Res. 2016 Oct 1;1648(Pt B):530-537. doi: 10.1016/j.brainres.2016.03.029. Epub 2016 Mar 26. Brain Res. 2016. PMID: 27021956 Review.
-
Structural insights into ISRIB, a memory-enhancing inhibitor of the integrated stress response.FEBS J. 2020 Jan;287(2):239-245. doi: 10.1111/febs.15073. Epub 2019 Nov 7. FEBS J. 2020. PMID: 31550413 Review.
Cited by
-
Attenuation of PKR-like ER Kinase (PERK) Signaling Selectively Controls Endoplasmic Reticulum Stress-induced Inflammation Without Compromising Immunological Responses.J Biol Chem. 2016 Jul 22;291(30):15830-40. doi: 10.1074/jbc.M116.738021. Epub 2016 May 23. J Biol Chem. 2016. PMID: 27226638 Free PMC article.
-
Defective regulation of the eIF2-eIF2B translational axis underlies depressive-like behavior in mice and correlates with major depressive disorder in humans.Transl Psychiatry. 2024 Oct 1;14(1):397. doi: 10.1038/s41398-024-03128-y. Transl Psychiatry. 2024. PMID: 39349438 Free PMC article.
-
Adaptive Protein Translation by the Integrated Stress Response Maintains the Proliferative and Migratory Capacity of Lung Adenocarcinoma Cells.Mol Cancer Res. 2019 Dec;17(12):2343-2355. doi: 10.1158/1541-7786.MCR-19-0245. Epub 2019 Sep 24. Mol Cancer Res. 2019. PMID: 31551255 Free PMC article.
-
Surviving and Adapting to Stress: Translational Control and the Integrated Stress Response.Antioxid Redox Signal. 2023 Aug;39(4-6):351-373. doi: 10.1089/ars.2022.0123. Epub 2023 May 9. Antioxid Redox Signal. 2023. PMID: 36943285 Free PMC article. Review.
-
Lung Injury Induces Alveolar Type 2 Cell Hypertrophy and Polyploidy with Implications for Repair and Regeneration.Am J Respir Cell Mol Biol. 2022 May;66(5):564-576. doi: 10.1165/rcmb.2021-0356OC. Am J Respir Cell Mol Biol. 2022. PMID: 35202558 Free PMC article.
References
-
- Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. - PubMed
-
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. - PubMed
-
- Halliday M, Mallucci GR. Targeting the unfolded protein response in neurodegeneration: a new approach to therapy. Neuropharmacology. 2014;76:169–174. - PubMed
-
- Hetz C, Mollereau B. Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci. 2014;15:233–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

