Chemoenzymatic synthesis of thiazolyl peptide natural products featuring an enzyme-catalyzed formal [4 + 2] cycloaddition
- PMID: 25742119
- PMCID: PMC4425689
- DOI: 10.1021/jacs.5b00940
Chemoenzymatic synthesis of thiazolyl peptide natural products featuring an enzyme-catalyzed formal [4 + 2] cycloaddition
Abstract
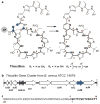
Thiocillins from Bacillus cereus ATCC 14579 are members of the well-known thiazolyl peptide class of natural product antibiotics, the biosynthesis of which has recently been shown to proceed via post-translational modification of ribosomally encoded precursor peptides. It has long been hypothesized that the final step of thiazolyl peptide biosynthesis involves a formal [4 + 2] cycloaddition between two dehydroalanines, a unique transformation that had eluded enzymatic characterization. Here we demonstrate that TclM, a single enzyme from the thiocillin biosynthetic pathway, catalyzes this transformation. To facilitate characterization of this new class of enzyme, we have developed a combined chemical and biological route to the complex peptide substrate, relying on chemical synthesis of a modified C-terminal fragment and coupling to a 38-residue leader peptide by means of native chemical ligation (NCL). This strategy, combined with active enzyme, provides a new chemoenzymatic route to this promising class of antibiotics.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Just-Baringo X, Albericio F, Alvarez M. Angew Chem, Int Ed. 2014;53:6602. - PubMed
-
- Nicolaou KC, Zou B, Dethe DH, Li DB, Chen DYK. Angew Chem, Int Ed. 2006;45:7786. - PubMed
-
- Nicolaou KC, Zak M, Safina BS, Estrada AA, Lee SH, Nevalainen M. J Am Chem Soc. 2005;127:11176. - PubMed
-
- Nicolaou KC, Safina BS, Zak M, Lee SH, Nevalainen M, Bella M, Estrada AA, Funke C, Zécri FJ, Bulat S. J Am Chem Soc. 2005;127:11159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

