Effect of mobile telephone reminders on treatment outcome in HIV: evidence from a randomised controlled trial in India
- PMID: 25742320
- PMCID: PMC4459037
- DOI: 10.1136/bmj.g5978
Effect of mobile telephone reminders on treatment outcome in HIV: evidence from a randomised controlled trial in India
Abstract
Objective: To assess whether customised mobile phone reminders would improve adherence to therapy and thus decrease virological failure among HIV infected patients starting antiretroviral treatment (ART).
Design: Randomised controlled trial among HIV infected patients initiating antiretroviral treatment.
Setting: Three diverse healthcare delivery settings in south India: two ambulatory clinics within the Indian national programme and one private HIV healthcare clinic.
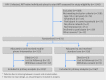
Participants: 631 HIV infected, ART naïve, adult patients eligible to initiate first line ART were randomly assigned to mobile phone intervention (n=315) or standard care (n=316) and followed for 96 weeks..
Intervention: The intervention consisted of customised, interactive, automated voice reminders, and a pictorial message that were sent weekly to the patients' mobile phones for the duration of the study.
Main outcome measures: The primary outcome was time to virological failure (viral load >400 copies/mL on two consecutive measurements). Secondary outcomes included ART adherence measured by pill count, death rate, and attrition rate. Suboptimal adherence was defined as mean adherence <95%.
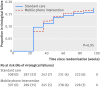
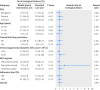
Results: Using an intention-to-treat approach we found no observed difference in time to virological failure between the allocation groups: failures in the intervention and standard care arms were 49/315 (15.6%) and 49/316 (15.5%) respectively (unadjusted hazard ratio 0.98, 95% confidence interval 0.67 to 1.47, P=0.95). The rate of virological failure in the intervention and standard care groups were 10.52 and 10.73 per 100 person years respectively. Comparison of suboptimal adherence was similar between both groups (unadjusted incidence rate ratio 1.24, 95% CI 0.93 to 1.65, P=0.14). Incidence proportion of patients with suboptimal adherence was 81/300 (27.0%) in the intervention arm and 65/299 (21.7%) in the standard care arm. The results of analyses adjusted for potential confounders were similar, indicating no significant difference between the allocation groups. Other secondary outcomes such as death and attrition rates, and subgroup analysis also showed comparable results across allocation groups.
Conclusions: In this multicentre randomised controlled trial among ART naïve patients initiating first line ART within the Indian national programme, we found no significant effect of the mobile phone intervention on either time to virological failure or ART adherence at the end of two years of therapy.Trial registration Current Controlled Trials ISRCTN79261738.
© Shet et al 2014.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
Comment in
-
Mobile phone messaging to improve health.BMJ. 2014 Oct 24;349:g6158. doi: 10.1136/bmj.g6158. BMJ. 2014. PMID: 25954987 No abstract available.
References
-
- Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet 2010; 376(9755): 1838-45. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
