Anemia: progress in molecular mechanisms and therapies
- PMID: 25742458
- PMCID: PMC4452951
- DOI: 10.1038/nm.3814
Anemia: progress in molecular mechanisms and therapies
Abstract
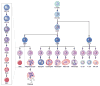
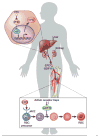
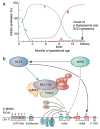
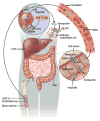
Anemia is a major source of morbidity and mortality worldwide. Here we review recent insights into how red blood cells (RBCs) are produced, the pathogenic mechanisms underlying various forms of anemia, and novel therapies derived from these findings. It is likely that these new insights, mainly arising from basic scientific studies, will contribute immensely to both the understanding of frequently debilitating forms of anemia and the ability to treat affected patients. Major worldwide diseases that are likely to benefit from new advances include the hemoglobinopathies (β-thalassemia and sickle cell disease); rare genetic disorders of RBC production; and anemias associated with chronic kidney disease, inflammation, and cancer. Promising new approaches to treatment include drugs that target recently defined pathways in RBC production, iron metabolism, and fetal globin-family gene expression, as well as gene therapies that use improved viral vectors and newly developed genome editing technologies.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

