Mitochondrial apoptosis: killing cancer using the enemy within
- PMID: 25742467
- PMCID: PMC4366906
- DOI: 10.1038/bjc.2015.85
Mitochondrial apoptosis: killing cancer using the enemy within
Abstract
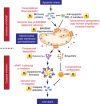
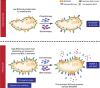
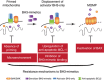
Apoptotic cell death inhibits oncogenesis at multiple stages, ranging from transformation to metastasis. Consequently, in order for cancer to develop and progress, apoptosis must be inhibited. Cell death also plays major roles in cancer treatment, serving as the main effector function of many anti-cancer therapies. In this review, we discuss the role of apoptosis in the development and treatment of cancer. Specifically, we focus upon the mitochondrial pathway of apoptosis-the most commonly deregulated form of cell death in cancer. In this process, mitochondrial outer membrane permeabilisation or MOMP represents the defining event that irrevocably commits a cell to die. We provide an overview of how this pathway is regulated by BCL-2 family proteins and describe ways in which cancer cells can block it. Finally, we discuss exciting new approaches aimed at specifically inducing mitochondrial apoptosis in cancer cells, outlining their potential pitfalls, while highlighting their considerable therapeutic promise.
Figures
References
-
- Aranovich A, Liu Q, Collins T, Geng F, Dixit S, Leber B, Andrews DW. Differences in the mechanisms of proapoptotic BH3 proteins binding to Bcl-XL and Bcl-2 quantified in live MCF-7 cells. Mol Cell. 2012;45 (6:754–763. - PubMed
-
- Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, Mc Henry KT, Pinchback RM, Ligon AH, Cho YJ, Haery L, Greulich H, Reich M, Winckler W, Lawrence MS, Weir BA, Tanaka KE, Chiang DY, Bass AJ, Loo A, Hoffman C, Prensner J, Liefeld T, Gao Q, Yecies D, Signoretti S, Maher E, Kaye FJ, Sasaki H, Tepper JE, Fletcher JA, Tabernero J, Baselga J, Tsao MS, Demichelis F, Rubin MA, Janne PA, Daly MJ, Nucera C, Levine RL, Ebert BL, Gabriel S, Rustgi AK, Antonescu CR, Ladanyi M, Letai A, Garraway LA, Loda M, Beer DG, True LD, Okamoto A, Pomeroy SL, Singer S, Golub TR, Lander ES, Getz G, Sellers WR, Meyerson M. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463 (7283:899–905. - PMC - PubMed
-
- Bonneau B, Prudent J, Popgeorgiev N, Gillet G. Non-apoptotic roles of Bcl-2 family: the calcium connection. Biochim Biophys Acta. 2013;1833 (7:1755–1765. - PubMed
-
- Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9 (5:351–365. - PubMed
-
- Clarke AR, Purdie CA, Harrison DJ, Morris RG, Bird CC, Hooper ML, Wyllie AH. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993;362 (6423:849–852. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

