Augmented BMP signaling in the neural crest inhibits nasal cartilage morphogenesis by inducing p53-mediated apoptosis
- PMID: 25742798
- PMCID: PMC4378250
- DOI: 10.1242/dev.118802
Augmented BMP signaling in the neural crest inhibits nasal cartilage morphogenesis by inducing p53-mediated apoptosis
Abstract
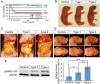
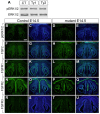
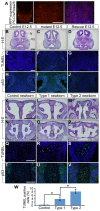
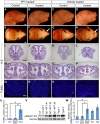
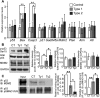
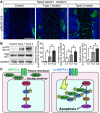
Bone morphogenetic protein (BMP) signaling plays many roles in skull morphogenesis. We have previously reported that enhanced BMP signaling through the BMP type IA receptor (BMPR1A) in cranial neural crest cells causes craniosynostosis during postnatal development. Additionally, we observed that 55% of Bmpr1a mutant mice show neonatal lethality characterized by a distended gastrointestinal tract. Here, we show that severely affected mutants exhibit defective nasal cartilage, failure of fusion between the nasal septum and the secondary palate, and higher levels of phosphorylated SMAD1 and SMAD5 in the nasal tissue. TUNEL demonstrated an increase in apoptosis in both condensing mesenchymal tissues and cartilage of the nasal region in mutants. The levels of p53 (TRP53) tumor suppressor protein were also increased in the same tissue. Injection of pifithrin-α, a chemical inhibitor of p53, into pregnant mice prevented neonatal lethality while concomitantly reducing apoptosis in nasal cartilage primordia, suggesting that enhanced BMP signaling induces p53-mediated apoptosis in the nasal cartilage. The expression of Bax and caspase 3, downstream targets of p53, was increased in the mutants; however, the p53 expression level was unchanged. It has been reported that MDM2 interacts with p53 to promote degradation. We found that the amount of MDM2-p53 complex was decreased in all mutants, and the most severely affected mutants had the largest decrease. Our previous finding that the BMP signaling component SMAD1 prevents MDM2-mediated p53 degradation coupled with our new data indicate that augmented BMP signaling induces p53-mediated apoptosis by prevention of p53 degradation in developing nasal cartilage. Thus, an appropriate level of BMP signaling is required for proper craniofacial morphogenesis.
Keywords: Apoptosis; BMP; Craniofacial development; MDM2; Nasal septum; Neural crest cell; SMAD; p53.
© 2015. Published by The Company of Biologists Ltd.
Figures


Similar articles
-
Reduced bone morphogenetic protein receptor type 1A signaling in neural-crest-derived cells causes facial dysmorphism.Dis Model Mech. 2012 Nov;5(6):948-55. doi: 10.1242/dmm.009274. Epub 2012 Jul 5. Dis Model Mech. 2012. PMID: 22773757 Free PMC article.
-
Augmentation of Smad-dependent BMP signaling in neural crest cells causes craniosynostosis in mice.J Bone Miner Res. 2013 Jun;28(6):1422-33. doi: 10.1002/jbmr.1857. J Bone Miner Res. 2013. PMID: 23281127 Free PMC article.
-
BmpR1A is a major type 1 BMP receptor for BMP-Smad signaling during skull development.Dev Biol. 2017 Sep 1;429(1):260-270. doi: 10.1016/j.ydbio.2017.06.020. Epub 2017 Jun 19. Dev Biol. 2017. PMID: 28641928 Free PMC article.
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
Common mechanisms in development and disease: BMP signaling in craniofacial development.Cytokine Growth Factor Rev. 2016 Feb;27:129-39. doi: 10.1016/j.cytogfr.2015.11.004. Epub 2015 Nov 24. Cytokine Growth Factor Rev. 2016. PMID: 26747371 Free PMC article. Review.
Cited by
-
Expression of Noggin and Gremlin1 and its implications in fine-tuning BMP activities in mouse cartilage tissues.J Orthop Res. 2017 Aug;35(8):1671-1682. doi: 10.1002/jor.23463. Epub 2016 Nov 8. J Orthop Res. 2017. PMID: 27769098 Free PMC article.
-
Signaling Mechanisms Underlying Genetic Pathophysiology of Craniosynostosis.Int J Biol Sci. 2019 Jan 1;15(2):298-311. doi: 10.7150/ijbs.29183. eCollection 2019. Int J Biol Sci. 2019. PMID: 30745822 Free PMC article. Review.
-
Phenotypic Analyses of Genetically Modified Mice for BMP Receptors.Methods Mol Biol. 2019;1891:179-189. doi: 10.1007/978-1-4939-8904-1_13. Methods Mol Biol. 2019. PMID: 30414133 Free PMC article.
-
BMP Signaling in the Development and Regeneration of Cranium Bones and Maintenance of Calvarial Stem Cells.Front Cell Dev Biol. 2020 Mar 10;8:135. doi: 10.3389/fcell.2020.00135. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32211409 Free PMC article. Review.
-
Genetic background dependent modifiers of craniosynostosis severity.J Struct Biol. 2020 Dec 1;212(3):107629. doi: 10.1016/j.jsb.2020.107629. Epub 2020 Sep 23. J Struct Biol. 2020. PMID: 32976998 Free PMC article.
References
-
- Anderson R. M., Lawrence A. R., Stottmann R. W., Bachiller D. and Klingensmith J. (2002). Chordin and noggin promote organizing centers of forebrain development in the mouse. Development 129, 4975-4987. - PubMed
-
- Badoux D. M. (1966). Framed structures in the mammalian skull. Acta Morphol. Neerlando Scandinavica 6, 239-250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

