Challenge to the suppression of tumor growth by the β4-galactosyltransferase genes
- PMID: 25743061
- PMCID: PMC4405391
- DOI: 10.2183/pjab.91.1
Challenge to the suppression of tumor growth by the β4-galactosyltransferase genes
Abstract
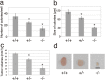
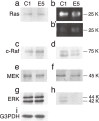
It has been well established that structural changes in glycans attached to proteins and lipids are associated with malignant transformation of cells. We focused on galactose residues among the sugars since they are involved in the galectin-mediated biology, and many carbohydrate antigens are frequently expressed on this sugar. We found changes in the expression of the β4-galactosyltransferase (β4GalT) 2 and 5 genes in cancer cells: decreased expression of the β4GalT2 gene and increased expression of the β4GalT5 gene. The growth of mouse melanoma cells showing enhanced expression of the β4GalT2 gene or reduced expression of the β4GalT5 gene is inhibited remarkably in syngeneic mice. Tumor growth inhibition is probably caused by the induction of apoptosis, inhibition of angiogenesis, and/or reduced MAPK signals. Direct transduction of human β4GalT2 cDNA together with the adenovirus vector into human hepatocellular carcinoma cells grown in SCID mice results in marked growth retardation of the tumors. β4GalT gene-transfer appears to be a potential tool for cancer therapy.
Figures








Similar articles
-
Enhanced expression of the β4-galactosyltransferase 2 gene impairs mammalian tumor growth.Cancer Gene Ther. 2014 Jun;21(6):219-27. doi: 10.1038/cgt.2014.21. Epub 2014 Jun 6. Cancer Gene Ther. 2014. PMID: 24903013
-
Gene expression levels of β4-galactosyltransferase 5 correlate with the tumorigenic potentials of B16-F10 mouse melanoma cells.Glycobiology. 2014 Jun;24(6):532-41. doi: 10.1093/glycob/cwu021. Epub 2014 Mar 20. Glycobiology. 2014. PMID: 24653215
-
Downregulation of Transcription Factor Sp1 Suppresses Malignant Properties of A549 Human Lung Cancer Cell Line with Decreased β4-Galactosylation of Highly Branched N-Glycans.Biol Pharm Bull. 2017 Aug 1;40(8):1282-1288. doi: 10.1248/bpb.b17-00212. Epub 2017 May 20. Biol Pharm Bull. 2017. PMID: 28529241
-
[Regulation of human β-1,4-galactosyltransferase V gene expression in cancer cells].Yakugaku Zasshi. 2012;132(6):691-7. doi: 10.1248/yakushi.132.691. Yakugaku Zasshi. 2012. PMID: 22687727 Review. Japanese.
-
[Suppression of tumor growth by the β4-galactosyltransferase gene].Seikagaku. 2015 Jun;87(3):373-7. Seikagaku. 2015. PMID: 26571604 Review. Japanese. No abstract available.
Cited by
-
The Immunological Regulation Roles of Porcine β-1, 4 Galactosyltransferase V (B4GALT5) in PRRSV Infection.Front Cell Infect Microbiol. 2018 Mar 1;8:48. doi: 10.3389/fcimb.2018.00048. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29546034 Free PMC article.
References
-
- Abercrombie C., Ambrose E.J. (1962) The surface properties of cancer cells: A review. Cancer Res. 22, 525–548. - PubMed
-
- Sanfor B.H. (1967) An alteration in tumor histocompatibility induced by neuraminidase. Transplantation 5, 1273–1279. - PubMed
-
- Lin L.H., Stern J.L., Davisdon E.A. (1982) Clones from cultured B16-mouse melanoma cells resistant to wheat germ agglutinin and with altered production of mucin-type glycoproteins. Carbohydr. Res. 111, 257–271. - PubMed
-
- Meezan E., Wu H.C., Black P.H., Robbins P.W. (1969) Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. II. Separation of glycoproteins and glycopeptides by Sephadex chromatography. Biochemistry 8, 2518–2524. - PubMed
-
- Buck C.A., Glick M.C., Warren L. (1970) A comparative study of glycoproteins from the surface of control and Rous sarcoma virus transformed hamster cells. Biochemistry 9, 4567–4576. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

