Histological characterization of human breast implant capsules
- PMID: 25743110
- PMCID: PMC4434852
- DOI: 10.1007/s00266-014-0439-7
Histological characterization of human breast implant capsules
Erratum in
-
Erratum to: Histological Characterization of Human Breast Implant Capsules.Aesthetic Plast Surg. 2015 Jun;39(3):316-7. doi: 10.1007/s00266-015-0473-0. Aesthetic Plast Surg. 2015. PMID: 25877324 Free PMC article. No abstract available.
Abstract
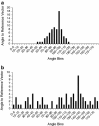
Background: This study investigated the relationships between histomorphological aspects of breast capsules, including capsule thickness, collagen fiber alignment, the presence of α-smooth muscle actin (α-SMA)-positive myofibroblasts, and clinical observations of capsular contracture.
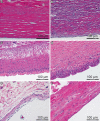
Methods: Breast capsule samples were collected at the time of implant removal in patients undergoing breast implant replacement or revision surgery. Capsular contracture was scored preoperatively using the Baker scale. Histological analysis included hematoxylin and eosin staining, quantitative analysis of capsule thickness, collagen fiber alignment, and immunohistochemical evaluation for α-SMA and CD68.
Results: Forty-nine samples were harvested from 41 patients. A large variation in histomorphology was observed between samples, including differences in cellularity, fiber density and organization, and overall structure. Baker I capsules were significantly thinner than Baker II, III, and IV capsules. Capsule thickness positively correlated with implantation time for all capsules and for contracted capsules (Baker III and IV). Contracted capsules had significantly greater collagen fiber alignment and α-SMA-positive immunoreactivity than uncontracted capsules (Baker I and II). Capsules from textured implants had significantly less α-SMA-positive immunoreactivity than capsules from smooth implants.
Conclusion: The histomorphological diversity observed between the breast capsules highlights the challenges of identifying mechanistic trends in capsular contracture. Our findings support the role of increasing capsule thickness and collagen fiber alignment, and the presence of contractile myofibroblasts in the development of contracture. These changes in capsule structure may be directly related to palpation stiffness considered in the Baker score. Approaches to disrupt these processes may aid in decreasing capsular contracture rates.
Level of evidence iii: This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266 .
Figures






References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

