Intraperitoneal administration of butyrate prevents the severity of acetic acid colitis in rats
- PMID: 25743124
- PMCID: PMC4357372
- DOI: 10.1631/jzus.B1400191
Intraperitoneal administration of butyrate prevents the severity of acetic acid colitis in rats
Abstract
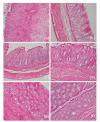
Intrarectal infusion of butyrate improves colorectal disorders including ulcerative colitis (UC). However, it is not established whether systemically administered butyrate benefits such patients. The current study aimed at exploring and comparing the potential of intraperitoneally, intrarectally, and orally administered butyrate against acetic acid (AA)-induced UC in rats. Intrarectal administration of 2 ml of 50% AA was done after or without prior treatment of rats for 7 consecutive days with 100 mg/kg sodium butyrate (SB) intraperitoneally, intrarectally, or orally. Rats were sacrificed after 48 h of AA-treatment. Subsequently, colon sections were processed routinely for histopathological examination. We clinically observed diarrhea, loose stools, and hemoccult-positive stools, and histologically, epithelial loss and ulceration, crypt damage, goblet cell depletion, hemorrhage, and mucosal infiltration of inflammatory cells. The changes were significantly reduced by intraperitoneal, intrarectal, or oral butyrate, with intraperitoneal butyrate exhibiting the highest potency. It is concluded that intraperitoneal administration of butyrate abrogates the lesions of AA-induced UC and its potency surpasses that of intrarectal or oral butyrate.
Keywords: Acetic acid; Butyrate; Intraperitoneal administration; Intrarectal administration; Oral administration; Ulcerative colitis.
Conflict of interest statement
All institutional and national guidelines for the care and use of laboratory animals were followed.
Figures



References
-
- Clark AJ. Absorption from the peritoneal cavity. J Pharmacol Exp Ther. 1921;16(6):415–433.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

