The role of sex hormones in immune protection of the female reproductive tract
- PMID: 25743222
- PMCID: PMC4716657
- DOI: 10.1038/nri3819
The role of sex hormones in immune protection of the female reproductive tract
Abstract
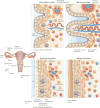
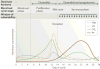
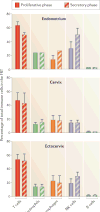
Within the human female reproductive tract (FRT), the challenge of protection against sexually transmitted infections (STIs) is coupled with the need to enable successful reproduction. Oestradiol and progesterone, which are secreted during the menstrual cycle, affect epithelial cells, fibroblasts and immune cells in the FRT to modify their functions and hence the individual's susceptibility to STIs in ways that are unique to specific sites in the FRT. The innate and adaptive immune systems are under hormonal control, and immune protection in the FRT varies with the phase of the menstrual cycle. Immune protection is dampened during the secretory phase of the cycle to optimize conditions for fertilization and pregnancy, which creates a 'window of vulnerability' during which potential pathogens can enter and infect the FRT.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- World Health Organization. Progress Report 2011: Global HIV/AIDS Response. WHO: 2011.
-
- World Health Organization. Prevalance and Incidence of Selected Sexually Transmitted Infections. WHO: 2011.
-
- Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22:1909–1917. This paper hypothesizes the existence of a window for increased susceptibility to HIV infection during the secretory phase of the menstrual cycle, during which time components of innate, humoral and cell-mediated immunity are suppressed by sex hormones. - PMC - PubMed
-
- Porterfield SP. In: Endocrine Physiology. Porterfield SP, editor. Mosby; 2001. pp. 169–199.
-
- Givan AL, et al. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol. 1997;38:350–359. This paper is the first and most complete side-by-side comparison of leukocyte subsets across the FRT during the proliferative and secretory phases of the menstrual cycle. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

