Vaginal Cytomegalovirus Shedding Before and After Initiation of Antiretroviral Therapy in Rakai, Uganda
- PMID: 25743428
- PMCID: PMC4548459
- DOI: 10.1093/infdis/jiv135
Vaginal Cytomegalovirus Shedding Before and After Initiation of Antiretroviral Therapy in Rakai, Uganda
Abstract
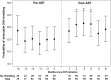
Vaginal shedding of cytomegalovirus (CMV) DNA was determined longitudinally among 96 women coinfected with human immunodeficiency virus (HIV), herpes simplex virus 2, and CMV starting antiretroviral therapy (ART) during a placebo-controlled trial of HSV-2 suppression with acyclovir in Rakai, Uganda. Vaginal CMV was detected in 75 of 96 women (78.0%) and 379 of 1080 individual visits (35.1%). ART status, higher HIV RNA viral load before ART initiation, and younger age were significantly associated with increased frequency of CMV shedding (P < .01). Compared to pre-ART, CMV shedding peaked from month 2 to month 4 after ART initiation, suggesting possible immune reconstitution inflammatory syndrome. Further studies need to determine the clinical significance of asymptomatic CMV shedding.
Keywords: Uganda; acyclovir; antiretroviral therapy (ART); cytomegalovirus (CMV); human immunodeficiency virus (HIV); immune reconstitution inflammatory syndrome (IRIS); reactivation.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Schoenfisch AL, Dollard SC, Amin M, et al. Cytomegalovirus (CMV) shedding is highly correlated with markers of immunosuppression in CMV-seropositive women. J Med Microbiol 2011; 60:768–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 7-UM1 AI068636-07/AI/NIAID NIH HHS/United States
- 1K23AI093152-01A1/AI/NIAID NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- UL1 TR000100/TR/NCATS NIH HHS/United States
- HHSN261200800001C/RC/CCR NIH HHS/United States
- K24 AI100665/AI/NIAID NIH HHS/United States
- R24AI106039/AI/NIAID NIH HHS/United States
- AI-30731-19/AI/NIAID NIH HHS/United States
- T32AI102623/AI/NIAID NIH HHS/United States
- K23 AI093152/AI/NIAID NIH HHS/United States
- P01 AI030731/AI/NIAID NIH HHS/United States
- P30 AI036214/AI/NIAID NIH HHS/United States
- T32 AI102623/AI/NIAID NIH HHS/United States
- AI100665/AI/NIAID NIH HHS/United States
- HHSN261200800001E/CA/NCI NIH HHS/United States
- UL1TR000100/TR/NCATS NIH HHS/United States
- P30-AI027763/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- R24 AI106039/AI/NIAID NIH HHS/United States

