Proteomic analyses of iron-responsive, Clp-dependent changes in Staphylococcus aureus
- PMID: 25743475
- PMCID: PMC4542840
- DOI: 10.1093/femspd/ftv004
Proteomic analyses of iron-responsive, Clp-dependent changes in Staphylococcus aureus
Abstract
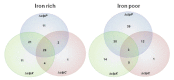
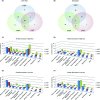
Staphylococcus aureus is a frequent human pathogen that is capable of causing a wide range of life-threatening infections. A promising antibacterial target is the Clp proteolytic system, which performs the vital function of maintaining protein turnover within the cell. This system primarily impacts the bacterial response to various stresses by degrading specific proteins but can also regulate a number of physiological processes through protein degradation. A critical stress to which S. aureus must adapt during infection of a vertebrate host is nutrient iron limitation. We have previously shown that the Clp system impacts expression of genes required for heme-iron acquisition during iron limitation and is required for staphylococcal infection. Based on these data, we sought to further define the Clp-dependent impact on S. aureus during iron limitation by characterizing the proteomic profiles of mutants inactivated for components of the Clp protease, including ClpP, ClpC and ClpX, in high- and low-iron conditions. Our results reveal numerous proteins altered in abundance in the clp mutants and provide new insights into the staphylococcal proteolytic network during nutrient iron limitation.
Keywords: 2D-DIGE; degradation; protein.
© FEMS 2015. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Brotz-Oesterhelt H, Beyer D, Kroll H-P, et al. Dysregulation of bacterial proteolytic machinery by a new class of antibiotics. Nat Med. 2005;11:1082–7. - PubMed
-
- Chatterjee I, Schmitt S, Batzilla CF, et al. Staphylococcus aureus ClpC ATPase is a late growth phase effector of metabolism and persistence. Proteomics. 2009;9:1152–76. - PubMed
-
- Feng J, Michalik S, Varming AN, et al. Trapping and proteomic identification of cellular substrates of the ClpP protease in Staphylococcus aureus. J Proteome Res. 2012;12:547–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

