Ancient Duplications and Expression Divergence in the Globin Gene Superfamily of Vertebrates: Insights from the Elephant Shark Genome and Transcriptome
- PMID: 25743544
- PMCID: PMC4476154
- DOI: 10.1093/molbev/msv054
Ancient Duplications and Expression Divergence in the Globin Gene Superfamily of Vertebrates: Insights from the Elephant Shark Genome and Transcriptome
Abstract
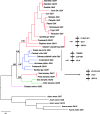
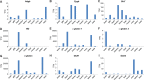
Comparative analyses of vertebrate genomes continue to uncover a surprising diversity of genes in the globin gene superfamily, some of which have very restricted phyletic distributions despite their antiquity. Genomic analysis of the globin gene repertoire of cartilaginous fish (Chondrichthyes) should be especially informative about the duplicative origins and ancestral functions of vertebrate globins, as divergence between Chondrichthyes and bony vertebrates represents the most basal split within the jawed vertebrates. Here, we report a comparative genomic analysis of the vertebrate globin gene family that includes the complete globin gene repertoire of the elephant shark (Callorhinchus milii). Using genomic sequence data from representatives of all major vertebrate classes, integrated analyses of conserved synteny and phylogenetic relationships revealed that the last common ancestor of vertebrates possessed a repertoire of at least seven globin genes: single copies of androglobin and neuroglobin, four paralogous copies of globin X, and the single-copy progenitor of the entire set of vertebrate-specific globins. Combined with expression data, the genomic inventory of elephant shark globins yielded four especially surprising findings: 1) there is no trace of the neuroglobin gene (a highly conserved gene that is present in all other jawed vertebrates that have been examined to date), 2) myoglobin is highly expressed in heart, but not in skeletal muscle (reflecting a possible ancestral condition in vertebrates with single-circuit circulatory systems), 3) elephant shark possesses two highly divergent globin X paralogs, one of which is preferentially expressed in gonads, and 4) elephant shark possesses two structurally distinct α-globin paralogs, one of which is preferentially expressed in the brain. Expression profiles of elephant shark globin genes reveal distinct specializations of function relative to orthologs in bony vertebrates and suggest hypotheses about ancestral functions of vertebrate globins.
Keywords: Chondrichthyes; gene duplication; gene family evolution; globin; myoglobin; neuroglobin.
© The Author 2015. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Awenius C, Hankeln T, Burmester T. Neuroglobins from the zebrafish Danio rerio and the pufferfish Tetraodon nigroviridis. Biochem Biophys Res Commun. 2001;287:418–421. - PubMed
-
- Bailly X, Vinogradov S. New York: Springer Verlag; 2008. The bilaterian sea urchin and the radial starlet sea anemone globins share strong homologies with vertebrate neuroglobins. In: Bolognesi M, di Prisco G, Verde C, editors. Dioxygen binding and sensing proteins; pp. 191–201.
-
- Blank M, Burmester T. Widespread occurrence of N-terminal acylation in animal globins and possible origin of respiratory globins from a membrane-bound ancestor. Mol Biol Evol. 2012;29:3553–3561. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

