Auditory mismatch impairments are characterized by core neural dysfunctions in schizophrenia
- PMID: 25743635
- PMCID: PMC5963408
- DOI: 10.1093/brain/awv049
Auditory mismatch impairments are characterized by core neural dysfunctions in schizophrenia
Abstract
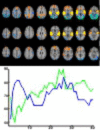
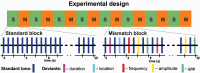
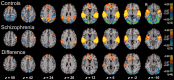
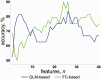
Major theories on the neural basis of schizophrenic core symptoms highlight aberrant salience network activity (insula and anterior cingulate cortex), prefrontal hypoactivation, sensory processing deficits as well as an impaired connectivity between temporal and prefrontal cortices. The mismatch negativity is a potential biomarker of schizophrenia and its reduction might be a consequence of each of these mechanisms. In contrast to the previous electroencephalographic studies, functional magnetic resonance imaging may disentangle the involved brain networks at high spatial resolution and determine contributions from localized brain responses and functional connectivity to the schizophrenic impairments. Twenty-four patients and 24 matched control subjects underwent functional magnetic resonance imaging during an optimized auditory mismatch task. Haemodynamic responses and functional connectivity were compared between groups. These data sets further entered a diagnostic classification analysis to assess impairments on the individual patient level. In the control group, mismatch responses were detected in the auditory cortex, prefrontal cortex and the salience network (insula and anterior cingulate cortex). Furthermore, mismatch processing was associated with a deactivation of the visual system and the dorsal attention network indicating a shift of resources from the visual to the auditory domain. The patients exhibited reduced activation in all of the respective systems (right auditory cortex, prefrontal cortex, and the salience network) as well as reduced deactivation of the visual system and the dorsal attention network. Group differences were most prominent in the anterior cingulate cortex and adjacent prefrontal areas. The latter regions also exhibited a reduced functional connectivity with the auditory cortex in the patients. In the classification analysis, haemodynamic responses yielded a maximal accuracy of 83% based on four features; functional connectivity data performed similarly or worse for up to about 10 features. However, connectivity data yielded a better performance when including more than 10 features yielding up to 90% accuracy. Among others, the most discriminating features represented functional connections between the auditory cortex and the anterior cingulate cortex as well as adjacent prefrontal areas. Auditory mismatch impairments incorporate major neural dysfunctions in schizophrenia. Our data suggest synergistic effects of sensory processing deficits, aberrant salience attribution, prefrontal hypoactivation as well as a disrupted connectivity between temporal and prefrontal cortices. These deficits are associated with subsequent disturbances in modality-specific resource allocation. Capturing different schizophrenic core dysfunctions, functional magnetic resonance imaging during this optimized mismatch paradigm reveals processing impairments on the individual patient level, rendering it a potential biomarker of schizophrenia.
Keywords: classification; functional magnetic resonance imaging; mismatch negativity; multivariate pattern analysis; schizophrenia.
© The Author (2015). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
Similar articles
-
Temporally anticorrelated brain networks during working memory performance reveal aberrant prefrontal and hippocampal connectivity in patients with schizophrenia.Prog Neuropsychopharmacol Biol Psychiatry. 2009 Nov 13;33(8):1464-73. doi: 10.1016/j.pnpbp.2009.07.032. Epub 2009 Aug 8. Prog Neuropsychopharmacol Biol Psychiatry. 2009. PMID: 19666074
-
Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia.Schizophr Res. 2010 Nov;123(2-3):105-15. doi: 10.1016/j.schres.2010.07.020. Epub 2010 Aug 17. Schizophr Res. 2010. PMID: 20724114
-
Intensive practice of a cognitive task is associated with enhanced functional integration in schizophrenia.Psychol Med. 2009 Nov;39(11):1809-19. doi: 10.1017/S0033291709005820. Epub 2009 Apr 20. Psychol Med. 2009. PMID: 19379537
-
[Functional MRI in schizophrenia. Diagnostics and therapy monitoring of cognitive deficits of schizophrenic patients by functional MRI].Radiologe. 2010 Feb;50(2):131-5. doi: 10.1007/s00117-009-1895-y. Radiologe. 2010. PMID: 20076939 Review. German.
-
Insular cortex and neuropsychiatric disorders: a review of recent literature.Eur Psychiatry. 2007 Sep;22(6):387-94. doi: 10.1016/j.eurpsy.2007.02.006. Epub 2007 Apr 9. Eur Psychiatry. 2007. PMID: 17416488 Review.
Cited by
-
Building Models of Functional Interactions Among Brain Domains that Encode Varying Information Complexity: A Schizophrenia Case Study.Neuroinformatics. 2022 Jul;20(3):777-791. doi: 10.1007/s12021-022-09563-w. Epub 2022 Mar 10. Neuroinformatics. 2022. PMID: 35267145 Free PMC article.
-
Impaired Subcortical Detection of Auditory Changes in Schizophrenia but Not in Major Depression.Schizophr Bull. 2020 Jan 4;46(1):193-201. doi: 10.1093/schbul/sbz027. Schizophr Bull. 2020. PMID: 31220318 Free PMC article.
-
Brain correlates of emotional prosodic change detection in autism spectrum disorder.Neuroimage Clin. 2020;28:102512. doi: 10.1016/j.nicl.2020.102512. Epub 2020 Nov 27. Neuroimage Clin. 2020. PMID: 33395999 Free PMC article.
-
Distinct Neuroanatomical Correlates of Neuropsychiatric Symptoms in the Three Main Forms of Genetic Frontotemporal Dementia in the GENFI Cohort.J Alzheimers Dis. 2018;65(1):147-163. doi: 10.3233/JAD-180053. J Alzheimers Dis. 2018. PMID: 30010122 Free PMC article.
-
mGluR5 binding changes during a mismatch negativity task in a multimodal protocol with [11C]ABP688 PET/MR-EEG.Transl Psychiatry. 2022 Jan 10;12(1):6. doi: 10.1038/s41398-021-01763-3. Transl Psychiatry. 2022. PMID: 35013095 Free PMC article.
References
-
- Baldeweg T, Klugman A, Gruzelier JH, Hirsch SR. Impairment in frontal but not temporal components of mismatch negativity in schizophrenia. Int J Psychophysiol. 2002;43:111–22. - PubMed
-
- Barbas H, Ghashghaei H, Dombrowski SM, Rempel-Clower NL. Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in the rhesus monkey. J Comp Neurol. 1999;410:343–67. - PubMed
-
- Belger A, Yucel GH, Donkers F. In search of psychosis biomarkers in high-risk populations: is the mismatch negativity the one we've been waiting for? Biol Psychiatry. 2012;71:94. - PubMed
-
- Benson PJ, Beedie SA, Shephard E, Giegling I, Rujescu D, St Clair D. Simple viewing tests can detect eye movement abnormalities that distinguish schizophrenia cases from controls with exceptional accuracy. Biol Psychiatry. 2012;72:716–24. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

