Neuronal NLRP1 inflammasome activation of Caspase-1 coordinately regulates inflammatory interleukin-1-beta production and axonal degeneration-associated Caspase-6 activation
- PMID: 25744023
- PMCID: PMC4563782
- DOI: 10.1038/cdd.2015.16
Neuronal NLRP1 inflammasome activation of Caspase-1 coordinately regulates inflammatory interleukin-1-beta production and axonal degeneration-associated Caspase-6 activation
Abstract
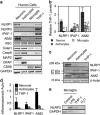
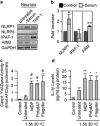
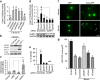
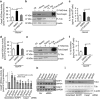
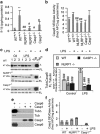
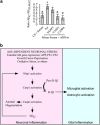
Neuronal active Caspase-6 (Casp6) is associated with Alzheimer disease (AD), cognitive impairment, and axonal degeneration. Caspase-1 (Casp1) can activate Casp6 but the expression and functionality of Casp1-activating inflammasomes has not been well-defined in human neurons. Here, we show that primary cultures of human CNS neurons expressed functional Nod-like receptor protein 1 (NLRP1), absent in melanoma 2, and ICE protease activating factor, but not the NLRP3, inflammasome receptor components. NLRP1 neutralizing antibodies in a cell-free system, and NLRP1 siRNAs in neurons hampered stress-induced Casp1 activation. NLRP1 and Casp1 siRNAs also abolished stress-induced Casp6 activation in neurons. The functionality of the NLRP1 inflammasome in serum-deprived neurons was also demonstrated by NLRP1 siRNA-mediated inhibition of speck formation of the apoptosis-associated speck-like protein containing a caspase recruitment domain conjugated to green fluorescent protein. These results indicated a novel stress-induced intraneuronal NLRP1/Casp1/Casp6 pathway. Lipopolysaccharide induced Casp1 and Casp6 activation in wild-type mice brain cortex, but not in that of Nlrp1(-/-) and Casp1(-/-) mice. NLRP1 immunopositive neurons were increased 25- to 30-fold in AD brains compared with non-AD brains. NLRP1 immunoreactivity in these neurons co-localized with Casp6 activity. Furthermore, the NLRP1/Casp1/Casp6 pathway increased amyloid beta peptide 42 ratio in serum-deprived neurons. Therefore, CNS human neurons express functional NLRP1 inflammasomes, which activate Casp1 and subsequently Casp6, thus revealing a fundamental mechanism linking intraneuronal inflammasome activation to Casp1-generated interleukin-1-β-mediated neuroinflammation and Casp6-mediated axonal degeneration.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

