Osteoblast-induced osteoclast apoptosis by fas ligand/FAS pathway is required for maintenance of bone mass
- PMID: 25744024
- PMCID: PMC4563780
- DOI: 10.1038/cdd.2015.14
Osteoblast-induced osteoclast apoptosis by fas ligand/FAS pathway is required for maintenance of bone mass
Abstract
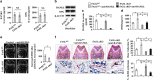
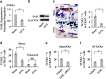
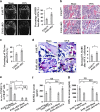
The interplay between osteoblasts and osteoclasts has a crucial role in maintaining bone homeostasis. In this study, we reveal that osteoblasts are capable of inducing osteoclast apoptosis by FAS ligand (FASL)/FAS signaling. Conditional knockout of FASL in osteoblasts results in elevated osteoclast numbers and activity, along with reduced bone mass, suggesting that osteoblast-produced FASL is required to maintain physiological bone mass. More interestingly, we show that osteoblasts from ovariectomized (OVX) osteoporotic mice exhibit decreased FASL expression that results from the IFN-γ- and TNF-α-activated NF-κB pathway, leading to reduced osteoclast apoptosis and increased bone resorption. Systemic administration of either IFN-γ or TNF-α ameliorates the osteoporotic phenotype in OVX mice and rescues FASL expression in osteoblasts. In addition, ovariectomy induces more significant bone loss in FASL conditional knockout mice than in control group with increased osteoclast activity in which the levels of RANKL and OPG remain unchanged. Taken together, this study suggests that osteoblast-induced osteoclast apoptosis via FASL/FAS signaling is a previously unrecognized mechanism that has an important role in the maintenance of bone mass in both physiological conditions and OVX osteoporosis.
Figures



Similar articles
-
Osteoblast-osteoclast interactions.Connect Tissue Res. 2018 Mar;59(2):99-107. doi: 10.1080/03008207.2017.1290085. Epub 2017 Mar 21. Connect Tissue Res. 2018. PMID: 28324674 Free PMC article. Review.
-
Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival.EMBO J. 2008 Feb 6;27(3):535-45. doi: 10.1038/sj.emboj.7601984. Epub 2008 Jan 24. EMBO J. 2008. PMID: 18219273 Free PMC article.
-
Osteoprotegerin Induces Apoptosis of Osteoclasts and Osteoclast Precursor Cells via the Fas/Fas Ligand Pathway.PLoS One. 2015 Nov 16;10(11):e0142519. doi: 10.1371/journal.pone.0142519. eCollection 2015. PLoS One. 2015. PMID: 26571489 Free PMC article.
-
IFN-γ directly inhibits TNF-α-induced osteoclastogenesis in vitro and in vivo and induces apoptosis mediated by Fas/Fas ligand interactions.Immunol Lett. 2011 Jun 30;137(1-2):53-61. doi: 10.1016/j.imlet.2011.02.017. Epub 2011 Feb 19. Immunol Lett. 2011. PMID: 21338623
-
Osteoblast-Osteoclast Communication and Bone Homeostasis.Cells. 2020 Sep 10;9(9):2073. doi: 10.3390/cells9092073. Cells. 2020. PMID: 32927921 Free PMC article. Review.
Cited by
-
Therapeutic Treatments for Osteoporosis-Which Combination of Pills Is the Best among the Bad?Int J Mol Sci. 2022 Jan 26;23(3):1393. doi: 10.3390/ijms23031393. Int J Mol Sci. 2022. PMID: 35163315 Free PMC article. Review.
-
Effect of α2‑macroglobulin in the early stage of jaw osteoradionecrosis.Int J Oncol. 2020 Jul;57(1):213-222. doi: 10.3892/ijo.2020.5051. Epub 2020 Apr 23. Int J Oncol. 2020. PMID: 32377713 Free PMC article.
-
Osteoblast-osteoclast interactions.Connect Tissue Res. 2018 Mar;59(2):99-107. doi: 10.1080/03008207.2017.1290085. Epub 2017 Mar 21. Connect Tissue Res. 2018. PMID: 28324674 Free PMC article. Review.
-
Cancer-educated mesenchymal stem cells promote the survival of cancer cells at primary and distant metastatic sites via the expansion of bone marrow-derived-PMN-MDSCs.Cell Death Dis. 2019 Dec 9;10(12):941. doi: 10.1038/s41419-019-2149-1. Cell Death Dis. 2019. PMID: 31819035 Free PMC article.
-
Expression of osteogenic factors in FasL-deficient calvarial cells.Physiol Res. 2023 Mar 8;72(1):117-121. doi: 10.33549/physiolres.934945. Epub 2022 Dec 22. Physiol Res. 2023. PMID: 36545877 Free PMC article.
References
-
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. - PubMed
-
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

