Bak apoptotic pores involve a flexible C-terminal region and juxtaposition of the C-terminal transmembrane domains
- PMID: 25744027
- PMCID: PMC4563781
- DOI: 10.1038/cdd.2015.15
Bak apoptotic pores involve a flexible C-terminal region and juxtaposition of the C-terminal transmembrane domains
Abstract
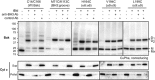
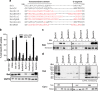
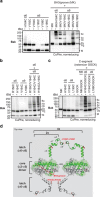
Bak and Bax mediate apoptotic cell death by oligomerizing and forming a pore in the mitochondrial outer membrane. Both proteins anchor to the outer membrane via a C-terminal transmembrane domain, although its topology within the apoptotic pore is not known. Cysteine-scanning mutagenesis and hydrophilic labeling confirmed that in healthy mitochondria the Bak α9 segment traverses the outer membrane, with 11 central residues shielded from labeling. After pore formation those residues remained shielded, indicating that α9 does not line a pore. Bak (and Bax) activation allowed linkage of α9 to neighboring α9 segments, identifying an α9:α9 interface in Bak (and Bax) oligomers. Although the linkage pattern along α9 indicated a preferred packing surface, there was no evidence of a dimerization motif. Rather, the interface was invoked in part by Bak conformation change and in part by BH3:groove dimerization. The α9:α9 interaction may constitute a secondary interface in Bak oligomers, as it could link BH3:groove dimers to high-order oligomers. Moreover, as high-order oligomers were generated when α9:α9 linkage in the membrane was combined with α6:α6 linkage on the membrane surface, the α6-α9 region in oligomerized Bak is flexible. These findings provide the first view of Bak carboxy terminus (C terminus) membrane topology within the apoptotic pore.
Figures




Similar articles
-
Pore formation by dimeric Bak and Bax: an unusual pore?Philos Trans R Soc Lond B Biol Sci. 2017 Aug 5;372(1726):20160218. doi: 10.1098/rstb.2016.0218. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28630157 Free PMC article. Review.
-
BAK α6 permits activation by BH3-only proteins and homooligomerization via the canonical hydrophobic groove.Proc Natl Acad Sci U S A. 2017 Jul 18;114(29):7629-7634. doi: 10.1073/pnas.1702453114. Epub 2017 Jul 3. Proc Natl Acad Sci U S A. 2017. PMID: 28673969 Free PMC article.
-
Assembly of the Bak apoptotic pore: a critical role for the Bak protein α6 helix in the multimerization of homodimers during apoptosis.J Biol Chem. 2013 Sep 6;288(36):26027-26038. doi: 10.1074/jbc.M113.490094. Epub 2013 Jul 26. J Biol Chem. 2013. PMID: 23893415 Free PMC article.
-
Disordered clusters of Bak dimers rupture mitochondria during apoptosis.Elife. 2017 Feb 6;6:e19944. doi: 10.7554/eLife.19944. Elife. 2017. PMID: 28182867 Free PMC article.
-
Bax, Bak and beyond - mitochondrial performance in apoptosis.FEBS J. 2018 Feb;285(3):416-431. doi: 10.1111/febs.14186. Epub 2017 Sep 4. FEBS J. 2018. PMID: 28755482 Review.
Cited by
-
The protective effect of soybean protein-derived peptides on apoptosis via the activation of PI3K-AKT and inhibition on apoptosis pathway.Food Sci Nutr. 2020 Jul 16;8(8):4591-4600. doi: 10.1002/fsn3.1776. eCollection 2020 Aug. Food Sci Nutr. 2020. PMID: 32884739 Free PMC article.
-
Pore formation by dimeric Bak and Bax: an unusual pore?Philos Trans R Soc Lond B Biol Sci. 2017 Aug 5;372(1726):20160218. doi: 10.1098/rstb.2016.0218. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28630157 Free PMC article. Review.
-
Bax transmembrane domain interacts with prosurvival Bcl-2 proteins in biological membranes.Proc Natl Acad Sci U S A. 2017 Jan 10;114(2):310-315. doi: 10.1073/pnas.1612322114. Epub 2016 Dec 27. Proc Natl Acad Sci U S A. 2017. PMID: 28028215 Free PMC article.
-
The BCL-2 arbiters of apoptosis and their growing role as cancer targets.Cell Death Differ. 2018 Jan;25(1):27-36. doi: 10.1038/cdd.2017.161. Epub 2017 Nov 3. Cell Death Differ. 2018. PMID: 29099483 Free PMC article. Review.
-
BAK α6 permits activation by BH3-only proteins and homooligomerization via the canonical hydrophobic groove.Proc Natl Acad Sci U S A. 2017 Jul 18;114(29):7629-7634. doi: 10.1073/pnas.1702453114. Epub 2017 Jul 3. Proc Natl Acad Sci U S A. 2017. PMID: 28673969 Free PMC article.
References
-
- Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. - PubMed
-
- Westphal D, Dewson G, Czabotar PE, Kluck RM. Molecular biology of Bax and Bak activation and action. Biochim Biophys Acta. 2011;1813:521–531. - PubMed
-
- Moldoveanu T, Liu Q, Tocilj A, Watson MH, Shore G, Gehring K. The x-ray structure of a BAK homodimer reveals an inhibitory zinc binding site. Mol Cell. 2006;24:677–688. - PubMed
-
- Suzuki M, Youle RJ, Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

