Oligoadenylate synthase-like (OASL) proteins: dual functions and associations with diseases
- PMID: 25744296
- PMCID: PMC4351405
- DOI: 10.1038/emm.2014.110
Oligoadenylate synthase-like (OASL) proteins: dual functions and associations with diseases
Abstract
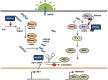
The study of antiviral pathways to reveal methods for the effective response and clearance of virus is closely related to understanding interferon (IFN) signaling and its downstream target genes, IFN-stimulated genes. One of the key antiviral factors induced by IFNs, 2'-5' oligoadenylate synthase (OAS), is a well-known molecule that regulates the early phase of viral infection by degrading viral RNA in combination with RNase L, resulting in the inhibition of viral replication. In this review, we describe OAS family proteins from a different point of view from that of previous reviews. We discuss not only RNase L-dependent (canonical) and -independent (noncanonical) pathways but also the possibility of the OAS family members as biomarkers for various diseases and clues to non-immunological functions based on recent studies. In particular, we focus on OASL, a member of the OAS family that is relatively less well understood than the other members. We will explain its anti- and pro-viral dual roles as well as the diseases related to single-nucleotide polymorphisms in the corresponding gene.
Figures
References
-
- Hovanessian AG, Wood JN. Anticellular and antiviral effects of pppA(2'p5'A)n. Virology. 1980;101:81–90. - PubMed
-
- Chebath J, Benech P, Revel M, Vigneron M. Constitutive expression of (2'-5') oligo A synthetase confers resistance to picornavirus infection. Nature. 1987;330:587–588. - PubMed
-
- Schroder HC, Suhadolnik RJ, Pfleiderer W, Charubala R, Muller WE. 2'-5')Oligoadenylate and intracellular immunity against retrovirus infection. Int J Biochem. 1992;24:55–63. - PubMed
-
- Kajaste-Rudnitski A, Mashimo T, Frenkiel MP, Guenet JL, Lucas M, Despres P. The 2',5'-oligoadenylate synthetase 1b is a potent inhibitor of West Nile virus replication inside infected cells. J Biol Chem. 2006;281:4624–4637. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

