Dose-dense temozolomide: is it still promising?
- PMID: 25744349
- PMCID: PMC4533399
- DOI: 10.2176/nmc.ra.2014-0277
Dose-dense temozolomide: is it still promising?
Abstract
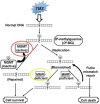
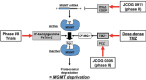
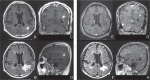
Glioblastoma (GBM) has proven to be incurable despite recent progress on its standard of care using temozolomide (TMZ) as the main trunk of initial therapy for newly diagnosed GBM. One of the main reasons accounting for the dismal prognosis is attributed to lack of active therapeutic regimens at recurrence. Since TMZ is the most active cytotoxic agent against GBM, and the standard dosing of TMZ has shown favorable safety profile in clinical trials, re-challenge with TMZ in increased dose density schedules for recurrent tumors that have evaded from prior standard TMZ therapy appears to be a rational approach and has been intensively exploited. A number of phase II clinical trials using different alternating scheduling of dose-dense TMZ (ddTMZ) have shown superior efficacy over the standard TMZ or historical controls with other alkylating agents including nitrosoureas and procarbazine. One ddTMZ schedule, consisting of a 21-days on/7-days off regimen was applied to newly-diagnosed GBM as the adjuvant monotherapy after completion of combined radiation and TMZ and failed to demonstrate survival benefit in a large phase III trial (RTOG 0525). Thus its role in TMZ-pretreated, recurrent GBM should be carefully pursuit in randomized trials, e.g., planned JCOG 1308 trial comparing a 7-days on/7-days off ddTMZ regimen used upfront at the first relapse followed by bevacizumab on progression versus bevacizumab alone, investigating whether insertion of ddTMZ prior to bevacizumab could bestow better outcome in the recurrent setting. In this article, mode of action, past trials, and future directions of ddTMZ therapy are discussed.
Conflict of interest statement
Dr. Motoo Nagane has received honoraria from Chugai Pharmaceutical Co., Ltd. (Tokyo). The author has registered online Self-reported COK Disclosure Statement Forms through the website for JNS members.
Figures
Republished in
-
Dose-dense Temozolomide: Is It Still Promising?Neurol Med Chir (Tokyo). 2015;55 Suppl 1:38-49. Neurol Med Chir (Tokyo). 2015. PMID: 26236801
References
-
- The Committee of Brain Tumor Registry of Japan : Report of Brain Tumor Registry of Japan (2001–2004) 13th Edition. Neurol Med Chir (Tokyo) 54 ( Suppl 1): 9– 102, 2014
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987– 996, 2005 - PubMed
-
- Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T: Bevacizumab plus radiotherapytemozolomide for newly diagnosed glioblastoma. N Engl J Med 370: 709– 722, 2014 - PubMed
-
- Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ, Mehta MP: A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370: 699– 708, 2014 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

