Pathophysiology, diagnosis, and treatment of radiation necrosis in the brain
- PMID: 25744350
- PMCID: PMC4533398
- DOI: 10.2176/nmc.ra.2014-0188
Pathophysiology, diagnosis, and treatment of radiation necrosis in the brain
Abstract
New radiation modalities have made it possible to prolong the survival of individuals with malignant brain tumors, but symptomatic radiation necrosis becomes a serious problem that can negatively affect a patient's quality of life through severe and lifelong effects. Here we review the relevant literature and introduce our original concept of the pathophysiology of brain radiation necrosis following the treatment of brain, head, and neck tumors. Regarding the pathophysiology of radiation necrosis, we introduce two major hypotheses: glial cell damage or vascular damage. For the differential diagnosis of radiation necrosis and tumor recurrence, we focus on the role of positron emission tomography. Finally, in accord with our hypothesis regarding the pathophysiology, we describe the promising effects of the anti-vascular endothelial growth factor antibody bevacizumab on symptomatic radiation necrosis in the brain.
Conflict of interest statement
There is no conflict of interest to disclose for any of the authors.
Figures


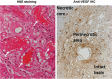
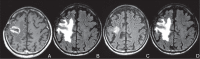
Republished in
-
Pathophysiology, Diagnosis, and Treatment of Radiation Necrosis in the Brain.Neurol Med Chir (Tokyo). 2015;55 Suppl 1:50-9. Neurol Med Chir (Tokyo). 2015. PMID: 26236802
References
-
- Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC: Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 77: 996– 1001, 2010. - PubMed
-
- Minniti G, D'Angelillo RM, Scaringi C, Trodella LE, Clarke E, Matteucci P, Osti MF, Ramella S, Enrici RM, Trodella L: Fractionated stereotactic radiosurgery for patients with brain metastases. J Neurooncol 117: 295– 301, 2014. - PubMed
-
- Telera S, Fabi A, Pace A, Vidiri A, Anelli V, Carapella CM, Marucci L, Crispo F, Sperduti I, Pompili A: Radionecrosis induced by stereotactic radiosurgery of brain metastases: results of surgery and outcome of disease. J Neurooncol 113: 313– 325, 2013. - PubMed
-
- Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ: Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9: 453– 461, 2008. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

