Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis
- PMID: 25744474
- PMCID: PMC5144106
- DOI: 10.1016/S1473-3099(14)71073-4
Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis
Abstract
Background: Human papillomavirus (HPV) vaccination programmes were first implemented in several countries worldwide in 2007. We did a systematic review and meta-analysis to assess the population-level consequences and herd effects after female HPV vaccination programmes, to verify whether or not the high efficacy reported in randomised controlled clinical trials are materialising in real-world situations.
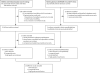
Methods: We searched the Medline and Embase databases (between Jan 1, 2007 and Feb 28, 2014) and conference abstracts for time-trend studies that analysed changes, between the pre-vaccination and post-vaccination periods, in the incidence or prevalence of at least one HPV-related endpoint: HPV infection, anogenital warts, and high-grade cervical lesions. We used random-effects models to derive pooled relative risk (RR) estimates. We stratified all analyses by age and sex. We did subgroup analyses by comparing studies according to vaccine type, vaccination coverage, and years since implementation of the vaccination programme. We assessed heterogeneity across studies using I(2) and χ(2) statistics and we did trends analysis to examine the dose-response association between HPV vaccination coverage and each study effect measure.
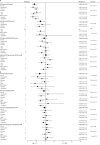
Findings: We identified 20 eligible studies, which were all undertaken in nine high-income countries and represent more than 140 million person-years of follow-up. In countries with female vaccination coverage of at least 50%, HPV type 16 and 18 infections decreased significantly between the pre-vaccination and post-vaccination periods by 68% (RR 0·32, 95% CI 0·19-0·52) and anogenital warts decreased significantly by 61% (0·39, 0·22-0·71) in girls 13-19 years of age. Significant reductions were also recorded in HPV types 31, 33, and 45 in this age group of girls (RR 0·72, 95% CI 0·54-0·96), which suggests cross-protection. Additionally, significant reductions in anogenital warts were also reported in boys younger than 20 years of age (0·66 [95% CI 0·47-0·91]) and in women 20-39 years of age (0·68 [95% CI 0·51-0·89]), which suggests herd effects. In countries with female vaccination coverage lower than 50%, significant reductions in HPV types 16 and 18 infection (RR 0·50, 95% CI 0·34-0·74]) and in anogenital warts (0·86 [95% CI 0·79-0·94]) occurred in girls younger than 20 years of age, with no indication of cross-protection or herd effects.
Interpretation: Our results are promising for the long-term population-level effects of HPV vaccination programmes. However, continued monitoring is essential to identify any signals of potential waning efficacy or type-replacement.
Funding: The Canadian Institutes of Health Research.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures




Comment in
-
Greatest effect of HPV vaccination from school-based programmes.Lancet Infect Dis. 2015 May;15(5):497-8. doi: 10.1016/S1473-3099(15)70078-2. Epub 2015 Mar 3. Lancet Infect Dis. 2015. PMID: 25744473 No abstract available.
-
HPV vaccine programmes: Current scenario and recommendations in India.Natl Med J India. 2016 Mar-Apr;29(2):91-3. Natl Med J India. 2016. PMID: 27586216 No abstract available.
References
-
- WHO . Countries with HPV vaccine in the national immunization programme and planned introductions. World Health Organization/IVB Database; Jan, 2014. [Sept 15, 2014]. http://www.who.int/immunization/diseases/hpv/decision_implementation/en/
-
- Cervical Cancer Action (CCA) Progress in cervical cancer prevention. [Sept 15, 2014];The CCA report card. 2012 Dec; http://www.cervicalcanceraction.org/pubs/CCA_reportcard_low-res.pdf.
-
- The World Bank [Sept 15, 2014];Countries and economies. 2006 http://data.worldbank.org/country.
-
- Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

