CK1δ restrains lipin-1 induction, lipid droplet formation and cell proliferation under hypoxia by reducing HIF-1α/ARNT complex formation
- PMID: 25744540
- PMCID: PMC4390155
- DOI: 10.1016/j.cellsig.2015.02.017
CK1δ restrains lipin-1 induction, lipid droplet formation and cell proliferation under hypoxia by reducing HIF-1α/ARNT complex formation
Abstract
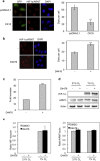
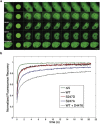
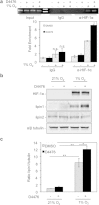
Proliferation of cells under hypoxia is facilitated by metabolic adaptation, mediated by the transcriptional activator Hypoxia Inducible Factor-1 (HIF-1). HIF-1α, the inducible subunit of HIF-1 is regulated by oxygen as well as by oxygen-independent mechanisms involving phosphorylation. We have previously shown that CK1δ phosphorylates HIF-1α in its N-terminus and reduces its affinity for its heterodimerization partner ARNT. To investigate the importance of this mechanism for cell proliferation under hypoxia, we visually monitored HIF-1α interactions within the cell nucleus using the in situ proximity ligation assay (PLA) and fluorescence recovery after photobleaching (FRAP). Both methods show that CK1δ-dependent modification of HIF-1α impairs the formation of a chromatin binding HIF-1 complex. This is confirmed by analyzing expression of lipin-1, a direct target of HIF-1 that mediates hypoxic neutral lipid accumulation. Inhibition of CK1δ increases lipid droplet formation and proliferation of both cancer and normal cells specifically under hypoxia and in an HIF-1α- and lipin-1-dependent manner. These data reveal a novel role for CK1δ in regulating lipid metabolism and, through it, cell adaptation to low oxygen conditions.
Keywords: CK1δ; HIF-1; Hypoxia; Lipid droplets; Lipid metabolism; Lipin1.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

