Hyperphosphatemia, Phosphoprotein Phosphatases, and Microparticle Release in Vascular Endothelial Cells
- PMID: 25745026
- PMCID: PMC4552113
- DOI: 10.1681/ASN.2014070642
Hyperphosphatemia, Phosphoprotein Phosphatases, and Microparticle Release in Vascular Endothelial Cells
Abstract
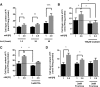
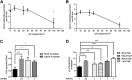
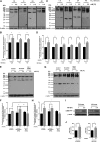
Hyperphosphatemia in patients with advanced CKD is thought to be an important contributor to cardiovascular risk, in part because of endothelial cell (EC) dysfunction induced by inorganic phosphate (Pi). Such patients also have an elevated circulating concentration of procoagulant endothelial microparticles (MPs), leading to a prothrombotic state, which may contribute to acute occlusive events. We hypothesized that hyperphosphatemia leads to MP formation from ECs through an elevation of intracellular Pi concentration, which directly inhibits phosphoprotein phosphatases, triggering a global increase in phosphorylation and cytoskeletal changes. In cultured human ECs (EAhy926), incubation with elevated extracellular Pi (2.5 mM) led to a rise in intracellular Pi concentration within 90 minutes. This was mediated by PiT1/slc20a1 Pi transporters and led to global accumulation of tyrosine- and serine/threonine-phosphorylated proteins, a marked increase in cellular Tropomyosin-3, plasma membrane blebbing, and release of 0.1- to 1-μm-diameter MPs. The effect of Pi was independent of oxidative stress or apoptosis. Similarly, global inhibition of phosphoprotein phosphatases with orthovanadate or fluoride yielded a global protein phosphorylation response and rapid release of MPs. The Pi-induced MPs expressed VE-cadherin and superficial phosphatidylserine, and in a thrombin generation assay, they displayed significantly more procoagulant activity than particles derived from cells incubated in medium with a physiologic level of Pi (1 mM). These data show a mechanism of Pi-induced cellular stress and signaling, which may be widely applicable in mammalian cells, and in ECs, it provides a novel pathologic link between hyperphosphatemia, generation of MPs, and thrombotic risk.
Keywords: CKD; cardiovascular disease; cell signaling; endothelial cells; hyperphosphatemia; microparticle.
Copyright © 2015 by the American Society of Nephrology.
Figures




Comment in
-
Chronic kidney disease: Procoagulant microparticles provide a novel pathogenic link between hyperphosphataemia and cardiovascular risk.Nat Rev Nephrol. 2015 May;11(5):256. doi: 10.1038/nrneph.2015.34. Epub 2015 Mar 24. Nat Rev Nephrol. 2015. PMID: 25802076 No abstract available.
References
-
- Foley RN, Parfrey PS, Sarnak MJ: Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol 9[Suppl]: S16–S23, 1998 - PubMed
-
- Ellam TJ, Chico TJ: Phosphate: The new cholesterol? The role of the phosphate axis in non-uremic vascular disease. Atherosclerosis 220: 310–318, 2012 - PubMed
-
- Di Marco GS, Hausberg M, Hillebrand U, Rustemeyer P, Wittkowski W, Lang D, Pavenstädt H: Increased inorganic phosphate induces human endothelial cell apoptosis in vitro. Am J Physiol Renal Physiol 294: F1381–F1387, 2008 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

