Structural biology. Structural basis for chemokine recognition and activation of a viral G protein-coupled receptor
- PMID: 25745166
- PMCID: PMC4445376
- DOI: 10.1126/science.aaa5026
Structural biology. Structural basis for chemokine recognition and activation of a viral G protein-coupled receptor
Abstract
Chemokines are small proteins that function as immune modulators through activation of chemokine G protein-coupled receptors (GPCRs). Several viruses also encode chemokines and chemokine receptors to subvert the host immune response. How protein ligands activate GPCRs remains unknown. We report the crystal structure at 2.9 angstrom resolution of the human cytomegalovirus GPCR US28 in complex with the chemokine domain of human CX3CL1 (fractalkine). The globular body of CX3CL1 is perched on top of the US28 extracellular vestibule, whereas its amino terminus projects into the central core of US28. The transmembrane helices of US28 adopt an active-state-like conformation. Atomic-level simulations suggest that the agonist-independent activity of US28 may be due to an amino acid network evolved in the viral GPCR to destabilize the receptor's inactive state.
Copyright © 2015, American Association for the Advancement of Science.
Figures

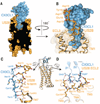
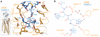
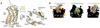
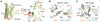

Comment in
-
Structural biology. Viral chemokine mimicry.Science. 2015 Mar 6;347(6226):1071-2. doi: 10.1126/science.aaa7998. Science. 2015. PMID: 25745149 No abstract available.
References
-
- Charo IF, Ransohoff RM. Engl. N. J. Med. 2006;354:610–621. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

