A universal infant rotavirus vaccine program in two delivery models: Effectiveness and adverse events following immunization
- PMID: 25746110
- PMCID: PMC4514393
- DOI: 10.1080/21645515.2015.1012028
A universal infant rotavirus vaccine program in two delivery models: Effectiveness and adverse events following immunization
Abstract
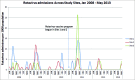
Rotavirus is the most common cause of diarrhea leading to hospitalization in young children. Rotavirus vaccines are available in Canada but have not been introduced in all provinces. In a controlled trial, 2 study sites (Prince Edward Island and the Capital District Health Authority (District 9, Nova Scotia) introduced universal rotavirus vaccine programs for infants at 2 and 4 months of age beginning 1 December 2010, using public health nurse or general practitioner-delivery models, respectively. A third site (Saint John, NB) served as the non-intervention control setting. Vaccine coverage, rotavirus hospitalizations, intussusception and all-cause diarrhea were monitored. A universal rotavirus vaccine program with >90% coverage was associated with reductions in rotavirus-associated hospitalizations (from a peak of 52.8 hospitalizations/100,000 population to 0 hospitalizations) in infants < 12 months and 1 to < 2 y of age 12 months after program implementation. No apparent reduction occurred in the site with vaccine coverage of < 40%, or in the non-intervention control site. No cases of intussusception were associated with vaccine receipt, and no increase in all-cause diarrhea was observed. A universal infant rotavirus vaccine program with high coverage was associated with reductions in rotavirus and no safety signals; no reduction was observed in settings with low vaccine coverage.
Keywords: Clinical Trials registration; NCT01273077; Rotavirus vaccines; immunization programs; public health practice.
Figures
Similar articles
-
Implementation of a universal rotavirus vaccination program: comparison of two delivery systems.BMC Public Health. 2014 Sep 2;14:908. doi: 10.1186/1471-2458-14-908. BMC Public Health. 2014. PMID: 25182067 Free PMC article. Clinical Trial.
-
Preparing for rotavirus vaccine introduction - A retrospective assessment of the epidemiology of intussusception in children below 2 years of age in Nepal.Vaccine. 2018 Dec 14;36(51):7836-7840. doi: 10.1016/j.vaccine.2017.11.022. Epub 2017 Nov 21. Vaccine. 2018. PMID: 29169894 Free PMC article.
-
Impact of a twelve-year rotavirus vaccine program on acute diarrhea mortality and hospitalization in Brazil: 2006-2018.Expert Rev Vaccines. 2020 Jun;19(6):585-593. doi: 10.1080/14760584.2020.1775081. Epub 2020 Jun 16. Expert Rev Vaccines. 2020. PMID: 32543244
-
Estimated reductions in hospitalizations and deaths from childhood diarrhea following implementation of rotavirus vaccination in Africa.Expert Rev Vaccines. 2017 Oct;16(10):987-995. doi: 10.1080/14760584.2017.1371595. Epub 2017 Sep 4. Expert Rev Vaccines. 2017. PMID: 28832219 Free PMC article. Review.
-
Rotavirus vaccination in Brazil: effectiveness and health impact seven years post-introduction.Expert Rev Vaccines. 2014 Jan;13(1):43-57. doi: 10.1586/14760584.2014.861746. Epub 2013 Dec 4. Expert Rev Vaccines. 2014. PMID: 24308577 Review.
Cited by
-
The impact of publicly funded rotavirus immunization programs on Canadian children.Can Commun Dis Rep. 2021 Mar 4;47(2):97-104. doi: 10.14745/ccdr.v47i02a02. eCollection 2021 Mar 4. Can Commun Dis Rep. 2021. PMID: 33746618 Free PMC article.
-
Assessing the risk of intussusception and rotavirus vaccine safety in Canada.Hum Vaccin Immunother. 2017 Mar 4;13(3):703-710. doi: 10.1080/21645515.2016.1240846. Epub 2016 Nov 11. Hum Vaccin Immunother. 2017. PMID: 27835525 Free PMC article.
-
Immunization in Canada: Update for 2015.J Can Chiropr Assoc. 2016 Mar;60(1):6-12. J Can Chiropr Assoc. 2016. PMID: 27069261 Free PMC article. No abstract available.
-
Population-Level Impact of Ontario's Infant Rotavirus Immunization Program: Evidence of Direct and Indirect Effects.PLoS One. 2016 May 11;11(5):e0154340. doi: 10.1371/journal.pone.0154340. eCollection 2016. PLoS One. 2016. PMID: 27168335 Free PMC article.
References
-
- Tate JE, Patel MM, Steele AD, Gentsch JR, Payne DC, Cortese MM, Nakagomi O, Cunliffe NA, Jiang B, Neuzil KM, et al. . Global impact of rotavirus vaccines. Expert Rev Vaccines 2010; 9(4):395-407; PMID:20370550; http://dx.doi.org/10.1586/erv.10.17 - DOI - PubMed
-
- Strategic Advisory Group of Vaccine Experts World Health Organization. Meeting of the immunization Strategic Advisory Group of Experts, April 2009 – conclusions and recommendations. Wkly Epidemiol Rec. 2009;84(23):220-36. - PubMed
-
- National Advisory Committee on Immunization. Statement on the recommended use of pentavalent human-bovine reassortant rotavirus vaccine. Can Commun Dis Rep. 2008;34(ACS-1):33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical