P21 activated kinase-1 mediates transforming growth factor β1-induced prostate cancer cell epithelial to mesenchymal transition
- PMID: 25746720
- PMCID: PMC4380670
- DOI: 10.1016/j.bbamcr.2015.02.023
P21 activated kinase-1 mediates transforming growth factor β1-induced prostate cancer cell epithelial to mesenchymal transition
Abstract
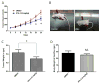
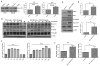
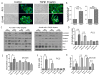
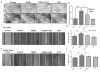
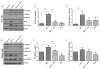
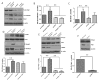
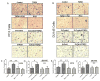
Transforming growth factor beta (TGFβ) is believed to play a dual role in prostate cancer. Molecular mechanism by which TGFβ1 suppresses early prostate tumor growth and induces epithelial-to-mesenchymal transition (EMT) in advanced stages is not known. We determined if P21-activated kinase1 (Pak1), which mediates cytoskeletal remodeling is necessary for the TGFβ1 induced prostate cancer EMT. Effects of TGFβ1 on control prostate cancer PC3 and DU145 cells and those with IPA 3 and siRNA mediated Pak1 inhibition were tested for prostate tumor xenograft in vivo and EMT in vitro. TGFβ1 inhibited PC3 tumor xenograft growth via activation of P38-MAPK and caspase-3, 9. Long-term stimulation with TGFβ1 induced PC3 and DU145 cell scattering and increased expression of EMT markers such as Snail and N-cadherin through tumor necrosis factor receptor-associated factor-6 (TRAF6)-mediated activation of Rac1/Pak1 pathway. Selective inhibition of Pak1 using IPA 3 or knockdown using siRNA both significantly inhibited TGFβ1-induced prostate cancer cell EMT and expression of mesenchymal markers. Our study demonstrated that TGFβ1 induces apoptosis and EMT in prostate cancer cells via activation of P38-MAPK and Rac1/Pak1 respectively. Our results reveal the potential therapeutic benefits of targeting TGFβ1-Pak1 pathway for advanced-stage prostate cancer.
Keywords: EMT; Pak1; Prostate cancer; Snail; TGFβ.
Copyright © 2015 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures

Similar articles
-
Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-β1/Smads signaling pathway mediated Snail/E-cadherin expression.BMC Cancer. 2015 Mar 5;15:97. doi: 10.1186/s12885-015-1119-y. BMC Cancer. 2015. PMID: 25884904 Free PMC article.
-
Rac1/PAK1 signaling promotes epithelial-mesenchymal transition of podocytes in vitro via triggering β-catenin transcriptional activity under high glucose conditions.Int J Biochem Cell Biol. 2013 Feb;45(2):255-64. doi: 10.1016/j.biocel.2012.11.003. Epub 2012 Nov 12. Int J Biochem Cell Biol. 2013. PMID: 23153508
-
Suppression of Akt1-β-catenin pathway in advanced prostate cancer promotes TGFβ1-mediated epithelial to mesenchymal transition and metastasis.Cancer Lett. 2017 Aug 28;402:177-189. doi: 10.1016/j.canlet.2017.05.028. Epub 2017 Jun 7. Cancer Lett. 2017. PMID: 28602980 Free PMC article.
-
Epithelial-Mesenchymal Transition (EMT) and Prostate Cancer.Adv Exp Med Biol. 2018;1095:101-110. doi: 10.1007/978-3-319-95693-0_6. Adv Exp Med Biol. 2018. PMID: 30229551 Review.
-
The role of Snail in prostate cancer.Cell Adh Migr. 2012 Sep-Oct;6(5):433-41. doi: 10.4161/cam.21687. Epub 2012 Sep 1. Cell Adh Migr. 2012. PMID: 23076049 Free PMC article. Review.
Cited by
-
p21-Activated Kinase: Role in Gastrointestinal Cancer and Beyond.Cancers (Basel). 2022 Sep 28;14(19):4736. doi: 10.3390/cancers14194736. Cancers (Basel). 2022. PMID: 36230657 Free PMC article. Review.
-
Unfractionated and Low Molecular Weight Heparin Reduce Platelet Induced Epithelial-Mesenchymal Transition in Pancreatic and Prostate Cancer Cells.Molecules. 2018 Oct 19;23(10):2690. doi: 10.3390/molecules23102690. Molecules. 2018. PMID: 30347648 Free PMC article.
-
Liposome-mediated delivery of the p21 activated kinase-1 (PAK-1) inhibitor IPA-3 limits prostate tumor growth in vivo.Nanomedicine. 2016 Jul;12(5):1231-1239. doi: 10.1016/j.nano.2016.01.003. Epub 2016 Mar 3. Nanomedicine. 2016. PMID: 26949163 Free PMC article.
-
Modulation in the microRNA repertoire is responsible for the stage-specific effects of Akt suppression on murine neuroendocrine prostate cancer.Heliyon. 2018 Sep 17;4(9):e00796. doi: 10.1016/j.heliyon.2018.e00796. eCollection 2018 Sep. Heliyon. 2018. PMID: 30238065 Free PMC article.
-
MicroRNAs as the critical regulators of cell migration and invasion in thyroid cancer.Biomark Res. 2022 Jun 4;10(1):40. doi: 10.1186/s40364-022-00382-4. Biomark Res. 2022. PMID: 35659780 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

