Mortality Caused by Bath Exposure of Zebrafish (Danio rerio) Larvae to Nervous Necrosis Virus Is Limited to the Fourth Day Postfertilization
- PMID: 25746990
- PMCID: PMC4407219
- DOI: 10.1128/AEM.04175-14
Mortality Caused by Bath Exposure of Zebrafish (Danio rerio) Larvae to Nervous Necrosis Virus Is Limited to the Fourth Day Postfertilization
Abstract
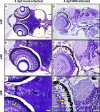
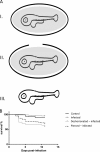
Nervous necrosis virus (NNV) is a member of the Betanodavirus genus that causes fatal diseases in over 40 species of fish worldwide. Mortality among NNV-infected fish larvae is almost 100%. In order to elucidate the mechanisms responsible for the susceptibility of fish larvae to NNV, we exposed zebrafish larvae to NNV by bath immersion at 2, 4, 6, and 8 days postfertilization (dpf). Here, we demonstrate that developing zebrafish embryos are resistant to NNV at 2 dpf due to the protection afforded by the egg chorion and, to a lesser extent, by the perivitelline fluid. The zebrafish larvae succumbed to NNV infection during a narrow time window around the 4th dpf, while 6- and 8-day-old larvae were much less sensitive, with mortalities of 24% and 28%, respectively.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




References
-
- Khan M, Khan S, Miyan K. 2011. Aquaculture as a food production system: a review. Biol Med 3:291–302.
-
- Munday BL, Kwang J, Moody N. 2002. Betanodavirus infections of teleost fish: a review. J Fish Dis 25:127–142. doi:10.1046/j.1365-2761.2002.00350.x. - DOI
-
- Tan C, Huang B, Chang SF, Ngoh GH, Munday B, Chen SC, Kwang J. 2001. Determination of the complete nucleotide sequences of RNA1 and RNA2 from greasy grouper (Epinephelus tauvina) nervous necrosis virus, Singapore strain. J Gen Virol 82:647–653. - PubMed
Publication types
MeSH terms
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

