γδ Intraepithelial Lymphocyte Migration Limits Transepithelial Pathogen Invasion and Systemic Disease in Mice
- PMID: 25747597
- PMCID: PMC4685713
- DOI: 10.1053/j.gastro.2015.02.053
γδ Intraepithelial Lymphocyte Migration Limits Transepithelial Pathogen Invasion and Systemic Disease in Mice
Abstract
Background & aims: Intraepithelial lymphocytes that express the γδ T-cell receptor (γδ IELs) limit pathogen translocation across the intestinal epithelium by unknown mechanisms. We investigated whether γδ IEL migration and interaction with epithelial cells promote mucosal barrier maintenance during enteric infection.
Methods: Salmonella typhimurium or Toxoplasma gondii were administered to knockout (KO) mice lacking either the T cell receptor δ chain (Tcrd) or CD103, or control TcrdEGFP C57BL/6 reporter mice. Intravital microscopy was used to visualize migration of green fluorescent protein (GFP)-tagged γδ T cells within the small intestinal mucosa of mice infected with DsRed-labeled S typhimurium. Mixed bone marrow chimeras were generated to assess the effects of γδ IEL migration on early pathogen invasion and chronic systemic infection.
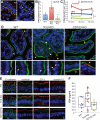
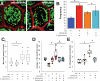
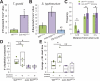
Results: Morphometric analyses of intravital video microscopy data showed that γδ IELs rapidly localized to and remained near epithelial cells in direct contact with bacteria. Within 1 hour, greater numbers of T gondii or S typhimurium were present within mucosae of mice with migration-defective occludin KO γδ T cells, compared with controls. Pathogen invasion in Tcrd KO mice was quantitatively similar to that in mice with occludin-deficient γδ T cells, whereas invasion in CD103 KO mice, which have increased migration of γδ T cells into the lateral intercellular space, was reduced by 63%. Consistent with a role of γδ T-cell migration in early host defense, systemic salmonellosis developed more rapidly and with greater severity in mice with occludin-deficient γδ IELs, relative to those with wild-type or CD103 KO γδ IELs.
Conclusions: In mice, intraepithelial migration to epithelial cells in contact with pathogens is essential to γδ IEL surveillance and immediate host defense. γδ IEL occludin is required for early surveillance that limits systemic disease.
Keywords: Host Defense; Intestinal Epithelium; T cell; Tight Junction.
Copyright © 2015 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Li C, Mannoor K, Inafuku M, et al. Protective function of an unconventional gammadelta T cell subset against malaria infection in apoptosis inhibitor deficient mice. Cell Immunol. 2012;279:151–9. - PubMed
-
- Dalton JE, Cruickshank SM, Egan CE, et al. Intraepithelial gammadelta+ lymphocytes maintain the integrity of intestinal epithelial tight junctions in response to infection. Gastroenterology. 2006;131:818–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000430/TR/NCATS NIH HHS/United States
- P30DK42086/DK/NIDDK NIH HHS/United States
- T32 HD007009/HD/NICHD NIH HHS/United States
- R21 AI094408/AI/NIAID NIH HHS/United States
- R56 DK094954/DK/NIDDK NIH HHS/United States
- P01 DK067887/DK/NIDDK NIH HHS/United States
- P30 DK042086/DK/NIDDK NIH HHS/United States
- K01DK093627/DK/NIDDK NIH HHS/United States
- F32DK084859/DK/NIDDK NIH HHS/United States
- P30CA14599/CA/NCI NIH HHS/United States
- U01AI77887/AI/NIAID NIH HHS/United States
- UL1 RR024999/RR/NCRR NIH HHS/United States
- P30 CA014599/CA/NCI NIH HHS/United States
- U19 AI095230/AI/NIAID NIH HHS/United States
- R01DK68271/DK/NIDDK NIH HHS/United States
- U19A1095230/PHS HHS/United States
- R01 DK061931/DK/NIDDK NIH HHS/United States
- R01 DK068271/DK/NIDDK NIH HHS/United States
- R01 HL118758/HL/NHLBI NIH HHS/United States
- R01DK61931/DK/NIDDK NIH HHS/United States
- K01 DK093627/DK/NIDDK NIH HHS/United States
- P30DK042086/DK/NIDDK NIH HHS/United States
- F32 DK084859/DK/NIDDK NIH HHS/United States
- U01 AI077887/AI/NIAID NIH HHS/United States
- R01HL118758/HL/NHLBI NIH HHS/United States
- UL1RR024999/RR/NCRR NIH HHS/United States
- S10 RR025643/RR/NCRR NIH HHS/United States
- R21A1094408/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

