Chasing cardiac physiology and pathology down the CaMKII cascade
- PMID: 25747749
- PMCID: PMC4436987
- DOI: 10.1152/ajpheart.00007.2015
Chasing cardiac physiology and pathology down the CaMKII cascade
Abstract
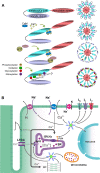
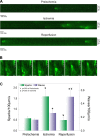
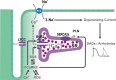
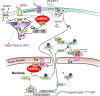
Calcium dynamics is central in cardiac physiology, as the key event leading to the excitation-contraction coupling (ECC) and relaxation processes. The primary function of Ca(2+) in the heart is the control of mechanical activity developed by the myofibril contractile apparatus. This key role of Ca(2+) signaling explains the subtle and critical control of important events of ECC and relaxation, such as Ca(2+) influx and SR Ca(2+) release and uptake. The multifunctional Ca(2+)-calmodulin-dependent protein kinase II (CaMKII) is a signaling molecule that regulates a diverse array of proteins involved not only in ECC and relaxation but also in cell death, transcriptional activation of hypertrophy, inflammation, and arrhythmias. CaMKII activity is triggered by an increase in intracellular Ca(2+) levels. This activity can be sustained, creating molecular memory after the decline in Ca(2+) concentration, by autophosphorylation of the enzyme, as well as by oxidation, glycosylation, and nitrosylation at different sites of the regulatory domain of the kinase. CaMKII activity is enhanced in several cardiac diseases, altering the signaling pathways by which CaMKII regulates the different fundamental proteins involved in functional and transcriptional cardiac processes. Dysregulation of these pathways constitutes a central mechanism of various cardiac disease phenomena, like apoptosis and necrosis during ischemia/reperfusion injury, digitalis exposure, post-acidosis and heart failure arrhythmias, or cardiac hypertrophy. Here we summarize significant aspects of the molecular physiology of CaMKII and provide a conceptual framework for understanding the role of the CaMKII cascade on Ca(2+) regulation and dysregulation in cardiac health and disease.
Keywords: Ca2+; CaMKII; arrhythmias; cell death; hypertrophy; ischemia/reperfusion.
Copyright © 2015 the American Physiological Society.
Figures


References
-
- Anderson ME. Multiple downstream proarrhythmic targets for calmodulin kinase II: moving beyond an ion channel-centric focus. Cardiovasc Res 73: 657–666, 2007. - PubMed
-
- Backs J, Olson EN. Control of cardiac growth by histone acetylation/deacetylation. Circ Res 98: 15–24, 2006. - PubMed
-
- Bai Y, Jones PP, Guo J, Zhong X, Clark RB, Zhou Q, Wang R, Vallmitjana A, Benitez R, Hove-Madsen L, Semeniuk L, Guo A, Song LS, Duff HJ, Chen SR. Phospholamban knockout breaks arrhythmogenic Ca2+ waves and suppresses catecholaminergic polymorphic ventricular tachycardia in mice. Circ Res 113: 517–526, 2013. - PMC - PubMed
-
- Bare DJ, Kettlun CS, Liang M, Bers DM, Mignery GA. Cardiac type 2 inositol 1,4,5-trisphosphate receptor: interaction and modulation by calcium/calmodulin-dependent protein kinase II. J Biol Chem 280: 15912–15920, 2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

