Uptake of systematic reviews and meta-analyses based on individual participant data in clinical practice guidelines: descriptive study
- PMID: 25747860
- PMCID: PMC4353308
- DOI: 10.1136/bmj.h1088
Uptake of systematic reviews and meta-analyses based on individual participant data in clinical practice guidelines: descriptive study
Abstract
Objective: To establish the extent to which systematic reviews and meta-analyses of individual participant data (IPD) are being used to inform the recommendations included in published clinical guidelines.
Design: Descriptive study.
Setting: Database maintained by the Cochrane IPD Meta-analysis Methods Group, supplemented by records of published IPD meta-analyses held in a separate database.
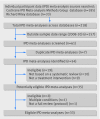
Population: A test sample of systematic reviews of randomised controlled trials that included a meta-analysis of IPD, and a separate sample of clinical guidelines, matched to the IPD meta-analyses according to medical condition, interventions, populations, and dates of publication.
Data extraction: Descriptive information on each guideline was extracted along with evidence showing use or critical appraisal, or both, of the IPD meta-analysis within the guideline; recommendations based directly on its findings and the use of other systematic reviews in the guideline.
Results: Based on 33 IPD meta-analyses and 177 eligible, matched clinical guidelines there was evidence that IPD meta-analyses were being under-utilised. Only 66 guidelines (37%) cited a matched IPD meta-analysis. Around a third of these (n=22, 34%) had critically appraised the IPD meta-analysis. Recommendations based directly on the matched IPD meta-analyses were identified for only 18 of the 66 guidelines (27%). For the guidelines that did not cite a matched IPD meta-analysis (n=111, 63%), search dates had preceded the publication of the IPD meta-analysis in 23 cases (21%); however, for the remainder, there was no obvious reasons why the IPD meta-analysis had not been cited.
Conclusions: Our results indicate that systematic reviews and meta-analyses based on IPD are being under-utilised. Guideline developers should routinely seek good quality and up to date IPD meta-analyses to inform guidelines. Increased use of IPD meta-analyses could lead to improved guidelines ensuring that routine patient care is based on the most reliable evidence available.
© Vale et al 2015.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
References
-
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. - PubMed
-
- Stewart L, Tierney J, Burdett S. Do systematic reviews based on individual patient data offer a means of circumventing biases associated with trial publications? In: Rothstein H, Sutton A, Borenstein M, eds. Publication bias in meta-analysis: prevention, assessment and adjustments. Chichester: Wiley, 2005:261-86.
-
- Tierney JF, Stewart LA. Investigating patient exclusion bias in meta-analysis. Int J Epidemiol 2005;34:79-87. - PubMed
-
- Burdett S, Stewart LA, Tierney JF. Publication bias and meta-analysis (a practical example). Int J Technol Assess Health Care 2003;19:129-34. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources