Current and future implications of basic and translational research on amyloid-β peptide production and removal pathways
- PMID: 25748120
- PMCID: PMC4503820
- DOI: 10.1016/j.mcn.2015.02.016
Current and future implications of basic and translational research on amyloid-β peptide production and removal pathways
Abstract
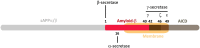
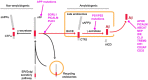
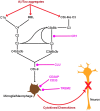
Inherited variants in multiple different genes are associated with increased risk for Alzheimer's disease (AD). In many of these genes, the inherited variants alter some aspect of the production or clearance of the neurotoxic amyloid β-peptide (Aβ). Thus missense, splice site or duplication mutants in the presenilin 1 (PS1), presenilin 2 (PS2) or the amyloid precursor protein (APP) genes, which alter the levels or shift the balance of Aβ produced, are associated with rare, highly penetrant autosomal dominant forms of Familial Alzheimer's Disease (FAD). Similarly, the more prevalent late-onset forms of AD are associated with both coding and non-coding variants in genes such as SORL1, PICALM and ABCA7 that affect the production and clearance of Aβ. This review summarises some of the recent molecular and structural work on the role of these genes and the proteins coded by them in the biology of Aβ. We also briefly outline how the emerging knowledge about the pathways involved in Aβ generation and clearance can be potentially targeted therapeutically. This article is part of Special Issue entitled "Neuronal Protein".
Keywords: APOE; APP; Abeta; Alzheimer; Amyloid; Dementia; EPHA1; Genetics; Neurodegeneration; Next generation sequencing; Nicastrin; PICALM; PSEN1; Presenilin; SORL1; Secretase; Tau; Vaccine.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Asai M., Hattori C., Szabo B., Sasagawa N., Maruyama K., Tanuma S., Ishiura S. Putative function of ADAM9, ADAM10, and ADAM17 as APP alpha-secretase. Biochem. Biophys. Res. Commun. 2003;301:231–235. - PubMed
-
- Ballatore C., Lee V.M.-Y., Trojanowski J.Q. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat. Rev. Neurosci. 2007;8:663–672. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

