Lung Mucosa Lining Fluid Modification of Mycobacterium tuberculosis to Reprogram Human Neutrophil Killing Mechanisms
- PMID: 25748325
- PMCID: PMC4548464
- DOI: 10.1093/infdis/jiv146
Lung Mucosa Lining Fluid Modification of Mycobacterium tuberculosis to Reprogram Human Neutrophil Killing Mechanisms
Abstract
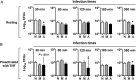
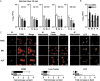
We have shown that human alveolar lining fluid (ALF) contains homeostatic hydrolases capable of altering the Mycobacterium tuberculosis cell wall and subsequently its interaction with human macrophages. Neutrophils are also an integral part of the host immune response to M. tuberculosis infection. Here we show that the human lung mucosa influences M. tuberculosis interaction with neutrophils, enhancing the intracellular killing of ALF-exposed M. tuberculosis and up-regulating the expression of tumor necrosis factor and interleukin 8. In contrast, ALF-exposed M. tuberculosis does not induce neutrophil apoptosis or necrosis, degranulation, or release of extracellular traps, and it decreases the oxidative response. These results suggest an important role for the human alveolar mucosa: increasing the innate capacity of the neutrophil to recognize and kill M. tuberculosis by favoring the use of intracellular mechanisms, while at the same time limiting neutrophil extracellular inflammatory responses to minimize their associated tissue damage.
Keywords: alveolar lining fluid; innate immunity; lung surfactant; neutrophil; tuberculosis.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Floyd K, Raviglione M. Global tuberculosis report 2013. http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf. Accessed 21 May 2014.
-
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 2006; 6:173–82. - PubMed
-
- Gonzalez-Cano P, Chacon-Salinas R, Ramos-Kichik V, et al. Double edge sword: the role of neutrophils in tuberculosis. In: Cardona PJ, ed. Understanding tuberculosis—analyzing the origin of Mycobacterium tuberculosis pathogenesis. Rijeka, Croatia: InTech, 2012:277–96.
-
- Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect 2003; 5:1317–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

