Phospholipase C and D regulation of Src, calcium release and membrane fusion during Xenopus laevis development
- PMID: 25748412
- PMCID: PMC4424143
- DOI: 10.1016/j.ydbio.2015.02.020
Phospholipase C and D regulation of Src, calcium release and membrane fusion during Xenopus laevis development
Abstract
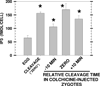
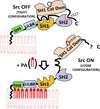
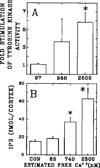
This review emphasizes how lipids regulate membrane fusion and the proteins involved in three developmental stages: oocyte maturation to the fertilizable egg, fertilization and during first cleavage. Decades of work show that phosphatidic acid (PA) releases intracellular calcium, and recent work shows that the lipid can activate Src tyrosine kinase or phospholipase C during Xenopus fertilization. Numerous reports are summarized to show three levels of increase in lipid second messengers inositol 1,4,5-trisphosphate and sn 1,2-diacylglycerol (DAG) during the three different developmental stages. In addition, possible roles for PA, ceramide, lysophosphatidylcholine, plasmalogens, phosphatidylinositol 4-phosphate, phosphatidylinositol 5-phosphate, phosphatidylinositol 4,5-bisphosphate, membrane microdomains (rafts) and phosphatidylinositol 3,4,5-trisphosphate in regulation of membrane fusion (acrosome reaction, sperm-egg fusion, cortical granule exocytosis), inositol 1,4,5-trisphosphate receptors, and calcium release are discussed. The role of six lipases involved in generating putative lipid second messengers during fertilization is also discussed: phospholipase D, autotaxin, lipin1, sphingomyelinase, phospholipase C, and phospholipase A2. More specifically, proteins involved in developmental events and their regulation through lipid binding to SH3, SH4, PH, PX, or C2 protein domains is emphasized. New models are presented for PA activation of Src (through SH3, SH4 and a unique domain), that this may be why the SH2 domain of PLCγ is not required for Xenopus fertilization, PA activation of phospholipase C, a role for PA during the calcium wave after fertilization, and that calcium/calmodulin may be responsible for the loss of Src from rafts after fertilization. Also discussed is that the large DAG increase during fertilization derives from phospholipase D production of PA and lipin dephosphorylation to DAG.
Keywords: Cleavage; Fertilization; Oocyte maturation; Phosphatidic acid; Phospholipase D; Protein–lipid binding; SH3 domain.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures



References
-
- Ajmat M, Bonilla F, Hermosilla P, Zelarayán L, Bühler M. Role of phospholipase A2 pathway in regulating activation of Bufo arenarum oocytes. Zygote. 2013;21:214–220. - PubMed
-
- Amata I, Maffei M, Igea A, Gay M, Vilaseca M, Nebreda AR, Pons M. Multi-phosphorylation of the Intrinsically Disordered Unique Domain of c-Src Studied by In-Cell and Real-Time NMR Spectroscopy. ChemBioChem. 2013;14:1820–1827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

